2.1.5: Spectrophotometry - Chemistry LibreTexts
Maybe your like
Devices and mechanism
Figure 1 illustrates the basic structure of spectrophotometers. It consists of a light source, a collimator, a monochromator, a wavelength selector, a cuvette for sample solution, a photoelectric detector, and a digital display or a meter. Detailed mechanism is described below. Figure 2 shows a sample spectrophotometer (Model: Spectronic 20D).
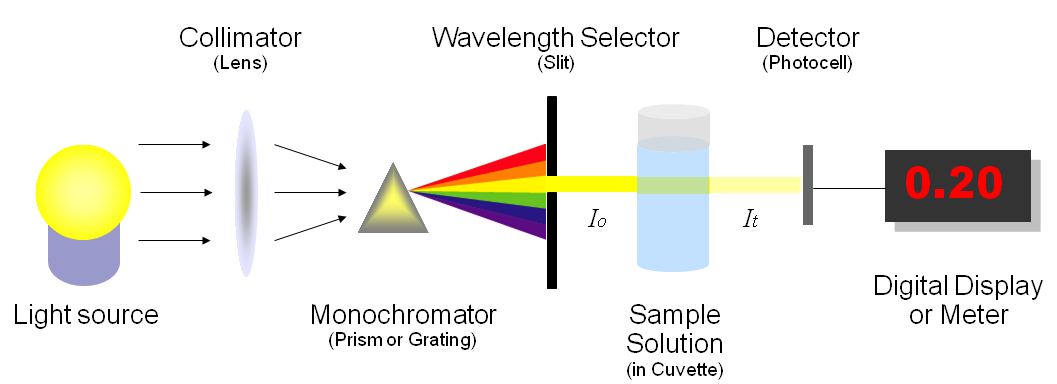
A spectrophotometer, in general, consists of two devices; a spectrometer and a photometer. A spectrometer is a device that produces, typically disperses and measures light. A photometer indicates the photoelectric detector that measures the intensity of light.
- Spectrometer: It produces a desired range of wavelength of light. First a collimator (lens) transmits a straight beam of light (photons) that passes through a monochromator (prism) to split it into several component wavelengths (spectrum). Then a wavelength selector (slit) transmits only the desired wavelengths, as shown in Figure 1.
- Photometer: After the desired range of wavelength of light passes through the solution of a sample in cuvette, the photometer detects the amount of photons that is absorbed and then sends a signal to a galvanometer or a digital display, as illustrated in Figure 1.

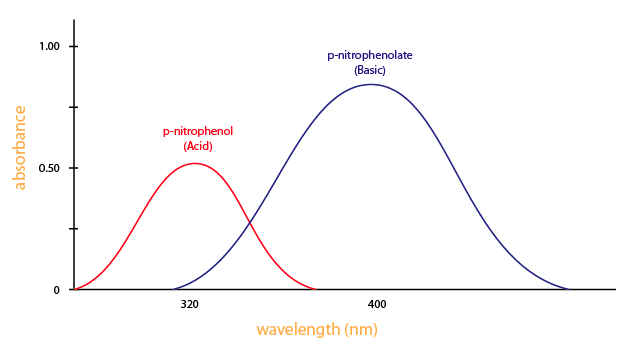
You need a spectrometer to produce a variety of wavelengths because different compounds absorb best at different wavelengths. For example, p-nitrophenol (acid form) has the maximum absorbance at approximately 320 nm and p-nitrophenolate (basic form) absorb best at 400nm, as shown in Figure 3.
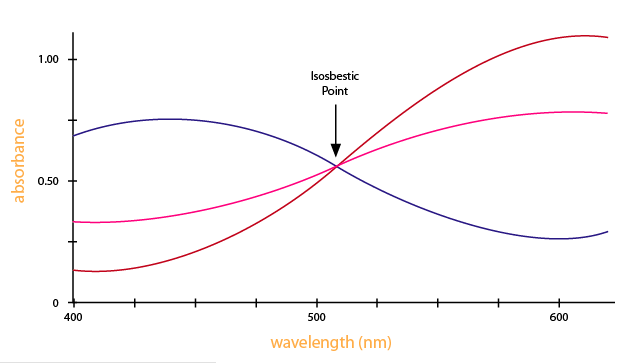
Looking at the graph that measures absorbance and wavelength, an isosbestic point can also be observed. An isosbestic point is the wavelength in which the absorbance of two or more species are the same. The appearance of an isosbestic point in a reaction demonstrates that an intermediate is NOT required to form a product from a reactant. Figure 4 shows an example of an isosbestic point.
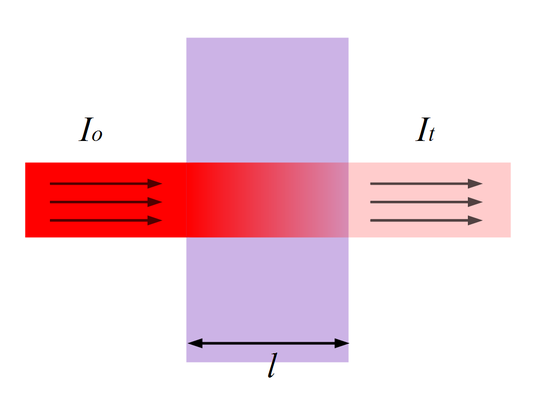
Referring back to Figure 1 (and Figure 5), the amount of photons that goes through the cuvette and into the detector is dependent on the length of the cuvette and the concentration of the sample. Once you know the intensity of light after it passes through the cuvette, you can relate it to transmittance (T). Transmittance is the fraction of light that passes through the sample. This can be calculated using the equation:
\(Transmittance (T) = \dfrac{I_t}{I_o}\)
Where It is the light intensity after the beam of light passes through the cuvette and Io is the light intensity before the beam of light passes through the cuvette. Transmittance is related to absorption by the expression:
\(Absorbance (A) = - log(T) = - log(\dfrac{I_t}{I_o})\)
Where absorbance stands for the amount of photons that is absorbed. With the amount of absorbance known from the above equation, you can determine the unknown concentration of the sample by using Beer-Lambert Law. Figure 5 illustrates transmittance of light through a sample. The length \(l\) is used for Beer-Lambert Law described below.
Tag » How Does A Spectrophotometer Work
-
How Does A Spectrophotometer Work?
-
Spectrophotometry - Tip Biosystems
-
How Does A Spectrophotometer Work? - Excedr
-
How A Spectrophotometer Works And Its Design - CRAIC Technologies
-
How Does A Spectrophotometer Work? - YouTube
-
How Does A Spectrophotometer Work? - YouTube
-
What Is A Spectrophotometer And How Does It Work?
-
What Wikipedia Can't Tell You About How Does A ... - Linquip
-
What Goes On Inside A Spectrophotometer?
-
How Does A Spectrophotometer Work? - Socratic
-
How Does A Spectrometer Work? | Sciencing
-
Principle Of Spectrophotometer And Its Applications| Chemistry - Byju's
-
How Does A Spectrometer Work? - BWTek