2.7: Applications Of Electron Configurations: Valence Electrons And ...
Maybe your like
Electron Dot Structures
Electron dot structures surround the elemental symbol of an element with one dot for every valence electron that the element contains. When drawing an electron dot structure, three rules must be followed:
- The first dot can be placed on any "side" of the elemental symbol (top, bottom, left, or right).
- The first four dots must each be placed on their own "side" of the elemental symbol. In other words, if the first dot is placed on the top of the elemental symbol, the second dot can be placed on the bottom, left, or right of the symbol, but cannot be placed at the top, alongside the first dot.
- The final four dots can again be placed on any "side" of the elemental symbol, but must be arranged such that no more than two dots exist on any "side" of the elemental symbol.
Again, consider sulfur, which has 6 valence electrons.
The elemental symbol for sulfur is S. Since an electron dot structure surrounds an elemental symbol with one dot for every valence electron that the element contains, sulfur's elemental symbol must be surrounded by 6 dots. Based on the rules given above, the dot representing sulfur's first valence electron can be placed on any "side" of the symbol, as shown below in Figure \(\PageIndex{1}\).
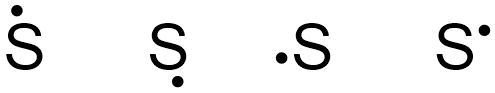
If the first structure in Figure \(\PageIndex{1}\) is chosen as the basis of sulfur's electron dot structure, the dot representing sulfur's second valence electron can be placed on the bottom, left, or right of the elemental symbol, but cannot be placed at the top, alongside the first dot. Figure \(\PageIndex{2}\) shows three structures with acceptable placements for sulfur's first two valence electrons, as well as a structure with an incorrect electron arrangement.
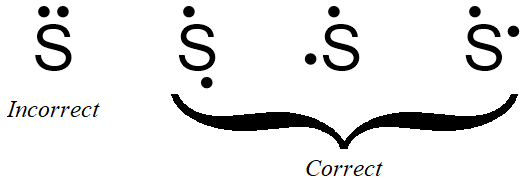
If the final structure in Figure \(\PageIndex{2}\) is chosen as the basis of sulfur's electron dot structure, the dots representing sulfur's third and fourth valence electrons must be placed on the bottom and to the left of the elemental symbol, but cannot be placed at the top or to the right of the elemental symbol. Figure \(\PageIndex{3}\) shows the only structure with an acceptable placement for sulfur's first four valence electrons.
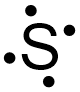
The dots representing sulfur's fifth and sixth valence electrons can again be placed on any "side" of the elemental symbol, but cannot both be placed on the same "side," so that no more than two dots exist on any "side" of the elemental symbol. Figure \(\PageIndex{4}\) shows all of the structures with acceptable placements for sulfur's six valence electrons. Therefore, any of the structures in Figure \(\PageIndex{4}\) is a valid electron dot structure for sulfur.
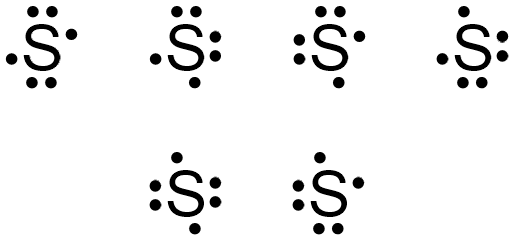
Example \(\PageIndex{3}\)
Draw a valid electron dot structure for nitrogen.
Solution
The elemental symbol for nitrogen is N. Based on Example \(\PageIndex{1}\) and Example \(\PageIndex{2}\), nitrogen has 5 valence electrons. Based on the rules described above, the first four dots must each be placed on their own "side" of the elemental symbol, and the fifth dot can be placed alongside any of the first four. Therefore, any of the following structures is a valid electron dot structure for nitrogen.

Exercise \(\PageIndex{3}\)
Draw a valid electron dot structure for each of the following elements.
- Neon
- Calcium


Tag » How Many Electrons Are In Sulfur
-
How Many Electrons Does Sulfur Have? - Quora
-
Sulfur: Orbital And Bonding Info
-
Electron Configuration For Sulfur (S) - TerpConnect
-
How To Find The Number Of Protons, Electrons, Neutrons For Sulfur (S)
-
#16 - Sulfur - S
-
How Many Electrons Are In A Sulfur Atom? A. 16 B. 47 C. 79 D. 38
-
How Many Electrons Are In Sulfur? | Homework.
-
How Many 'p' Electrons Are Present In A Sulphur Atom? - Vedantu
-
Sulfur - Element Information, Properties And Uses | Periodic Table
-
How Many Protons, Neutrons, Electrons Are In An Ion Of Sulfur (S^-2) ?
-
How Many Valence Electrons Are Present In Sulphur Atom? - Q&A
-
Expansion-of-octet-rule - Chemistry Guru
-
How Many Valence Electrons Does Sulfur(S) Have?
-
How Many Valence Electrons Are In An Atom Of Sulfur? - Toppr