4.3: Drawing Lewis Structures - Chemistry LibreTexts
Maybe your like
DRAWING LEWIS STRUCTURES
For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms. See these examples:
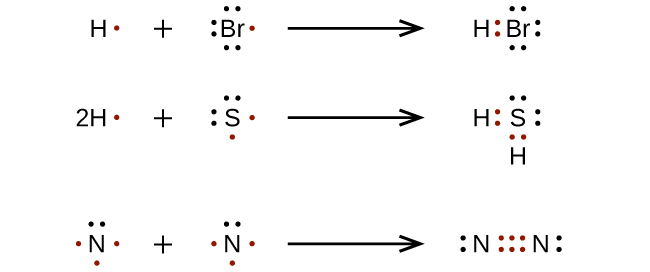
For more complicated molecules and molecular ions, it is helpful to follow the step-by-step procedure outlined here:
- Determine the total number of valence (outer shell) electrons among all the atoms. For cations, subtract one electron for each positive charge. For anions, add one electron for each negative charge.
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. (Generally, the least electronegative element should be placed in the center.) Connect each atom to the central atom with a single bond (one electron pair).
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom.
- Place all remaining electrons on the central atom.
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
Let us determine the Lewis structures of OF2 and HCN as examples in following this procedure:
1. Determine the total number of valence (outer shell) electrons in the molecule or ion. For a molecule, we add the number of valence electrons (use the main group number) on each atom in the molecule. This is the total number of electrons that must be used in the Lewis structure.
O + 2 (F) = OF2
6e- + (2 x 7e-) = 20e-
H + C + N = HCN
1e-+ 4e-+ 5e-= 10e-
2. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Note that H and F can only form one bond, and are always on the periphery rather than the central atom.
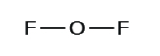
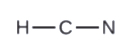
3. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
- In OF2, six electrons are placed on each F.
- In HCN, six electrons placed on N
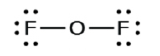
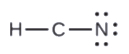
4. Place all remaining electrons on the central atom.
- In OF2, 4 electrons are placed on O.
- In HCN: no electrons remain (the total valence of 10e-is reached) so nothing changes.
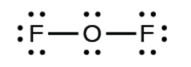
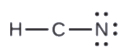
5. Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
- In OF2, each atom has an octet as drawn, so nothing changes.
- In HCN, form two more C–N bonds

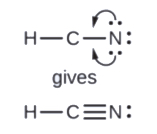
Finally, check to see if the total number of valence electrons are present in the Lewis structure. And then, inspect if the H atom has 2 electrons surrounding it and if each of the main group atoms is surrounded by 8 electrons.
Tag » How To Draw Lewis Dot Structure
-
How To Draw A Lewis Structure - ThoughtCo
-
Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures
-
How To Draw Lewis Structures - YouTube
-
Lewis Dot Structures | ChemTalk
-
Learn How To Draw Lewis Structures
-
Ch 1 : Drawing Lewis Structures - Chemistry - University Of Calgary
-
How To Draw A Lewis Structure - Science Notes
-
Lewis Electron Dot Structures - Detailed Explanation With Examples ...
-
Drawing Lewis Diagrams (video) - Khan Academy
-
Lewis Dot Structures — Rules & Examples - Expii
-
How To Draw Lewis Dot Structures (Easiest Method) - Chad's Prep®
-
[PDF] Drawing Lewis Dot Structures - East Central College
-
Electron Dot Structure Study Guide - Inspirit