5.11: Quantum Mechanical Atomic Model - Chemistry LibreTexts
Maybe your like
Quantum Mechanical Atomic Model
In 1926, Austrian physicist Erwin Schrödinger (1887-1961) used the wave-particle duality of the electron to develop and solve a complex mathematical equation that accurately described the behavior of the electron in a hydrogen atom. The quantum mechanical model of the atom comes from the solution to Schrödinger's equation. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the Bohr model, in which quantization was simply assumed with no mathematical basis.
Recall that in the Bohr model, the exact path of the electron was restricted to very well-defined circular orbits around the nucleus. The quantum mechanical model is a radical departure from that. Solutions to the Schrödinger wave equation, called wave functions, give only the probability of finding an electron at a given point around the nucleus. Electrons do not travel around the nucleus in simple circular orbits.
The location of the electrons in the quantum mechanical model of the atom is often referred to as an electron cloud. The electron cloud can be thought of in the following way: Imagine placing a square piece of paper on the floor with a dot in the circle representing the nucleus. Now take a marker and drop it onto the paper repeatedly, making small marks at each point the marker hits. If you drop the marker many, many times, the overall pattern of dots will be roughly circular. If you aim toward the center reasonably well, there will be more dots near the nucleus and progressively fewer dots as you move away from it. Each dot represents a location where the electron could be at any given moment. Because of the uncertainty principle, there is no way to know exactly where the electron is. An electron cloud has variable densities: a high density where the electron is most likely to be and a low density where the electron is least likely to be (see below).
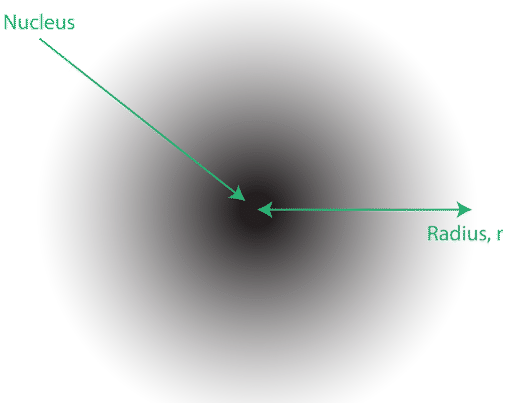
In order to specifically define the shape of the cloud, it is customary to refer to the area within which there is a \(90%\) chance of finding the electron. This is called an orbital, the three-dimensional region of space that indicates where there is a high probability of finding an electron.
Tag » How Did Schrodinger Discover The Electron Cloud
-
Electron Cloud Atomic Model | CK-12 Foundation
-
What Is The Electron Cloud Model? - Universe Today
-
Atomic Theory - Electron "cloud" - Math Science Nucleus
-
Development Of The Atomic Theory
-
Erwin Schrodinger
-
Electron Cloud Model, Theory & Examples
-
How Did Erwin Schrodinger Discover The Electron Cloud?
-
Electron Cloud - Simple English Wikipedia, The Free Encyclopedia
-
Modern Quantum Model: Schrodinger And Chadwick
-
Electron Cloud Model - Science Struck
-
What Is An Electron Cloud? - ThoughtCo
-
The Quantum Mechanical Model Of The Atom (article) - Khan Academy
-
Erwin Schrödinger – Facts
-
2.6: Orbitals, Electron Clouds, Probabilities, And Energies