8.6: Resonance Structures - Chemistry LibreTexts
Maybe your like
Ozone (\(O_3\))
1. We know that ozone has a V-shaped structure, so one O atom is central:
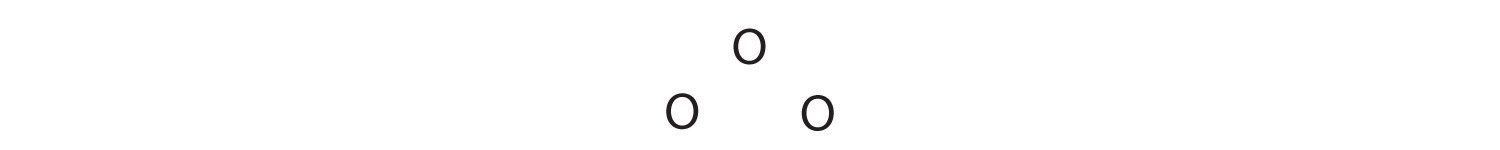
2. Each O atom has 6 valence electrons, for a total of 18 valence electrons.
3. Assigning one bonding pair of electrons to each oxygen–oxygen bond gives
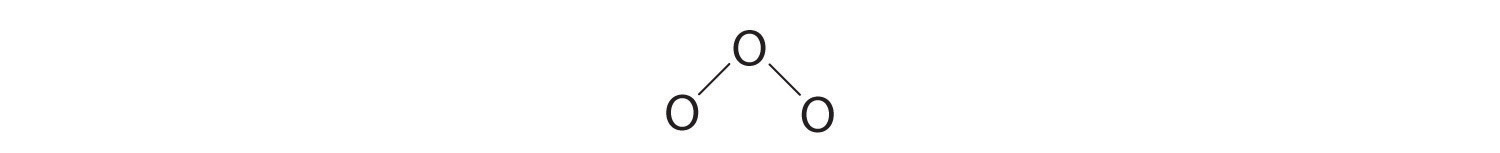
with 14 electrons left over.
4. If we place three lone pairs of electrons on each terminal oxygen, we obtain

and have 2 electrons left over.
5. At this point, both terminal oxygen atoms have octets of electrons. We therefore place the last 2 electrons on the central atom:
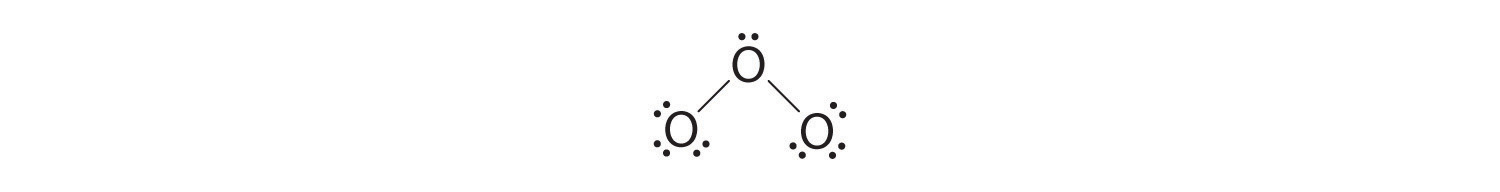
6. The central oxygen has only 6 electrons. We must convert one lone pair on a terminal oxygen atom to a bonding pair of electrons—but which one? Depending on which one we choose, we obtain either
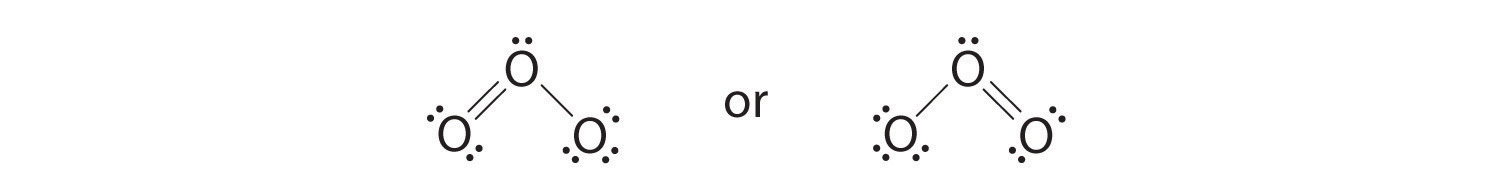
Which is correct? In fact, neither is correct. Both predict one O–O single bond and one O=O double bond. As you will learn, if the bonds were of different types (one single and one double, for example), they would have different lengths. It turns out, however, that both O–O bond distances are identical, 127.2 pm, which is shorter than a typical O–O single bond (148 pm) and longer than the O=O double bond in O2 (120.7 pm).
Equivalent Lewis dot structures, such as those of ozone, are called resonance structures. The position of the atoms is the same in the various resonance structures of a compound, but the position of the electrons is different. Double-headed arrows link the different resonance structures of a compound:
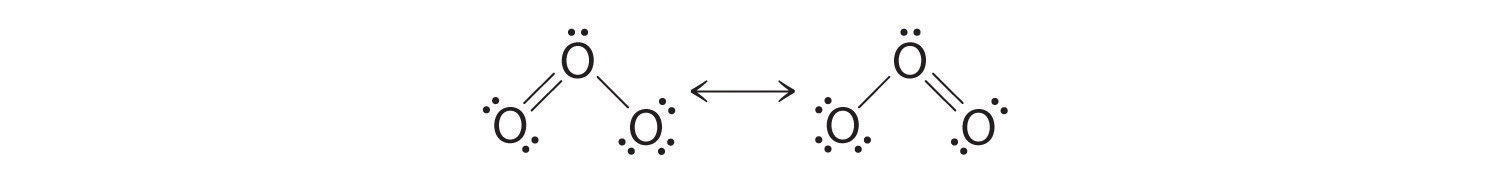
The double-headed arrow indicates that the actual electronic structure is an average of those shown, not that the molecule oscillates between the two structures.
When it is possible to write more than one equivalent resonance structure for a molecule or ion, the actual structure is the average of the resonance structures.
Tag » What Is A Resonance Structure
-
Resonance - Chemistry LibreTexts
-
Resonance Structures - Resonance Effect & Explanation ... - Byju's
-
Resonance Structures - YouTube
-
Ch 1 : Resonance - Chemistry
-
Resonance (chemistry) - Wikipedia
-
Resonance Structures (video) - Khan Academy
-
Resonance Structures | ChemTalk
-
1.3 Resonance Structures – Organic Chemistry I
-
Resonance Structures | Definition, Examples, Diagrams - Toppr
-
Resonance Structures - Definition, Nitrite Ion, Nitrate Ion, Carbonate ...
-
Resonance Structures – Easy Hard Science
-
What Are Resonance Structures? + Drawing Curved Arrows
-
Resonance Chemistry: Meaning & Examples | StudySmarter