Carbon–hydrogen Bond - Wikipedia
Maybe your like
In chemistry, the carbon–hydrogen bond (C−H bond) is a chemical bond between carbon and hydrogen atoms that can be found in many organic compounds.[1] This bond is a covalent, single bond, meaning that carbon shares its outer valence electrons with up to four hydrogens. This completes both of their outer shells, making them stable.[2]
Carbon–hydrogen bonds have a bond length of about 1.09 Å (1.09 × 10−10 m) and a bond energy of about 413 kJ/mol (see table below). Using Pauling's scale—C (2.55) and H (2.2)—the electronegativity difference between these two atoms is 0.35. Because of this small difference in electronegativities, the C−H bond is generally regarded as being non-polar. In structural formulas of molecules, the hydrogen atoms are often omitted. Compound classes consisting solely of C−H bonds and C−C bonds are alkanes, alkenes, alkynes, and aromatic hydrocarbons. Collectively they are known as hydrocarbons.
In October 2016, astronomers reported that the very basic chemical ingredients of life—the carbon–hydrogen molecule (CH, or methylidyne radical), the carbon–hydrogen positive ion (CH+) and the carbon ion (C+)—are created, in large part, using energy from the ultraviolet light of nearby stars, rather than in other ways, such as turbulent events related to supernovae and young stars, as thought earlier.[3]
Bond length
[edit]The length of the carbonhydrogen bond varies slightly with the hybridisation of the carbon atom. A bond between a hydrogen atom and an sp2 hybridised carbon atom is about 0.6% shorter than between hydrogen and sp3 hybridised carbon. A bond between hydrogen and sp hybridised carbon is shorter still, about 3% shorter than sp3 C–H. This trend is illustrated by the molecular geometry of ethane, ethylene and acetylene.[citation needed]
| Molecule | Methane | Ethane | Ethylene | Acetylene |
|---|---|---|---|---|
| Formula | CH4 | C2H6 | C2H4 | C2H2 |
| Class | alkane | alkane | alkene | alkyne |
| Structure |  |  | 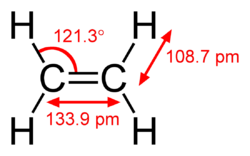 |  |
| Hybridisation of carbon | sp3 | sp3 | sp2 | sp |
| C–H bond length | 1.087 Å | 1.094 Å | 1.087 Å | 1.060 Å |
| Proportion of ethane C–H bond length | 99% | 100% | 99% | 97% |
| Structure determination method | microwave spectroscopy | microwave spectroscopy | microwave spectroscopy | infrared spectroscopy |
Reactions
[edit] Main article: Carbon–hydrogen bond activationThe C−H bond in general is very strong, so it is relatively unreactive. In several compound classes, collectively called carbon acids, the C−H bond can be sufficiently acidic for proton removal. Unactivated C−H bonds are found in alkanes and are not adjacent to a heteroatom (O, N, Si, etc.). Such bonds usually only participate in radical substitution. Many enzymes are known, however, to effect these reactions.[5]
Although the C−H bond is one of the strongest, it varies over 30% in magnitude for fairly stable organic compounds, even in the absence of heteroatoms.[6][7]
| Bond | Hydrocarbon radical | Molar Bond Dissociation Energy (kcal) | Molar Bond Dissociation Energy (kJ) |
|---|---|---|---|
| CH3−H | Methyl | 104 | 440 |
| C2H5−H | Ethyl | 98 | 410 |
| (CH3)2HC−H | Isopropyl | 95 | 400 |
| (CH3)3C−H | tert-Butyl | 93 | 390 |
| CH2=CH−H | vinyl | 112 | 470 |
| HC≡C−H | ethynyl | 133 | 560 |
| C6H5−H | phenyl | 110 | 460 |
| CH2=CHCH2−H | Allyl | 88 | 370 |
| C6H5CH2−H | Benzyl | 85 | 360 |
| OC4H7−H | tetrahydrofuranyl | 92 | 380 |
| CH3C(O)CH2−H | acetonyl | 96 | 400 |
See also
[edit]- Carbon–carbon bond
- Carbon–nitrogen bond
- Carbon–oxygen bond
- Carbon–fluorine bond
References
[edit]- ^ March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.
- ^ "Life Sciences Cyberbridge". Covalent Bonds. Archived from the original on 2015-09-18. Retrieved 2015-09-15.
- ^ Landau, Elizabeth (12 October 2016). "Building Blocks of Life's Building Blocks Come From Starlight". NASA. Retrieved 13 October 2016.
- ^ CRC Handbook of Chemistry and Physics, 88th edition
- ^ Bollinger, J. M. Jr., Broderick, J. B. "Frontiers in enzymatic C-H-bond activation" Current Opinion in Chemical Biology 2009, vol. 13, page 51-7. doi:10.1016/j.cbpa.2009.03.018
- ^ "Bond Energies". Organic Chemistry, Michigan State University. Archived from the original on 29 August 2016.
- ^ Yu-Ran Luo and Jin-Pei Cheng "Bond Dissociation Energies" in CRC Handbook of Chemistry and Physics, 96th Edition
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecules |
| |||||||||||||||||||
| Deuteratedmolecules |
| |||||||||||||||||||
| Unconfirmed |
| |||||||||||||||||||
| Related |
| |||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Legend |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tag » Why Are Ch Bonds Nonpolar
-
Why Are C-H Bonds Considered Nonpolar? - Quora
-
4.4: Polar And Non-polar Covalent Bonds - Chemistry LibreTexts
-
C-H Bond Polarity - CHEMISTRY COMMUNITY
-
Polar Covalent Bond - An Overview | ScienceDirect Topics
-
[PDF] Organic Chemistry (CHEM307)
-
Non-polar Covalent Bond - Definition, Examples, Formation - Byju's
-
Re: Why Is C-H Bond Non-polar, And O-H Bond Polar?
-
Chemical Bonds | Chemistry Of Life | Biology (article) - Khan Academy
-
Question Video: Identifying The Bond Type Of A C−H Bond - Nagwa
-
Polar And Non-Polar Covalent Bonds: Difference & List - StudySmarter
-
Types Of Covalent Bonds: Polar And Nonpolar
-
What Is A Nonpolar Covalent Bond? (Video) - Mometrix
-
Polar Vs. Non-Polar Bonds & Molecules - ChemTalk
-
C-H Bonds. Polar Or Non-polar? - Physics Forums
