CH103 – Chapter 8: Homeostasis And Cellular Function – Chemistry
Maybe your like
8.4 Solubility
The maximum amount of a substance that can be dissolved in a given volume of solvent is called solubility. Often, the solubility in water is expressed in gram/100 mL. A solution that has not reached its maximum solubility is called an unsaturated solution. This means that more solute could still be added to the solvent and dissolving would still occur.
A solution that has reached the maximum solubility is called a saturated solution. If more solute is added at this point, it will not dissolve into the solution. Instead it will remain precipitated as a solid at the bottom of the solution. Thus, one can often tell that a solution is saturated if extra solute is present (this can exist as another phase, such as gas, liquid, or solid). In a saturated solution there is no net change in the amount of solute dissolved, but the system is by no means static. In fact, the solute is constantly being dissolved and deposited at an equal rate. Such a phenomenon is called equilibrium. For example:
In special circumstances, a solution may be supersaturated. Supersaturated solutions are solutions that have dissolved solute beyond the normal saturation point. Usually a condition such as increased temperature or pressure is required to create a supersaturated solution. For example, sodium acetate has a very high solubility at 270 K. When cooled, such a solution stays dissolved in what is called a meta-stable state. However, when a seeding crystal is added to the solution, the extra solute will rapidly solidify. During the crystallization process, heat is evolved, and the solution becomes warm. Common hand warmers use this chemical process to generate heat.
Video showing the crystallization of a supersaturated solution of sodium acetate. Video by : North Carolina School of Science and Mathematics
So how can we predict the solubility of a substance?
One useful classification of materials is polarity. As you read about covalent and ionic compounds in Chapters 3 and 4, you learned that ionic compounds have the highest polarity forming full cations and anions within each molecule as electrons are donated from one atom to another. You also learned that covalent bonds could be polar or nonpolar in nature depending on whether or not the atoms involved in the bond share the electrons unequally or equally, respectively. Recall that the electronegativity difference can be used to determine the polarity of a substance. Typically an ionic bond has an electronegativity difference of 1.8 or above, whereas a polar covalent bond is between 0.4 to 1.8, and a nonpolar covalent bond is 0.4 or below.
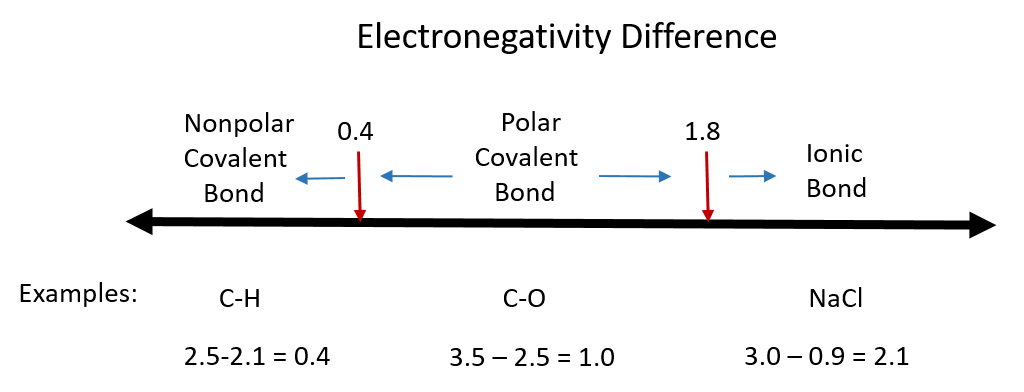
Figure 8.6 Electronegativity Difference Diagram. The diagram above is a guide for discerning what type of bond forms between two different atoms. By taking the difference between the electronegativity values for each of the atoms involved in the bond, the bond type and polarity can be predicted. Note that full ionic character is rarely reached, however when metals and nonmetals form bonds, they are named using the rules for ionic bonding.
Substances with zero or low electronegativity difference such as H2, O2, N2, CH4, CCl4 are nonpolar compounds, whereas H2O, NH3, CH3OH, NO, CO, HCl, H2S, PH3 higher electronegativity difference are polar compounds. Typically compounds that have similar polarity are soluble in one another. This can be described by the rule:
Like Dissolves Like.
This means that substances must have similar intermolecular forces to form solutions. When a soluble solute is introduced into a solvent, the particles of solute can interact with the particles of solvent. In the case of a solid or liquid solute, the interactions between the solute particles and the solvent particles are so strong that the individual solute particles separate from each other and, surrounded by solvent molecules, enter the solution. (Gaseous solutes already have their constituent particles separated, but the concept of being surrounded by solvent particles still applies.) This process is called solvation and is illustrated in Figure 7.2. When the solvent is water, the word hydration, rather than solvation, is used.
Tag » How Does A Cell Maintain Homeostasis
-
Homeostasis Role & Process | How Do Cells Maintain ...
-
How Do Cells Maintain Homeostasis - Biology Dictionary
-
Homeostasis
-
[PDF] Homeostasis And Transport - Organisms Must Maintain Internal ...
-
[PDF] Homeostasis And Transport Lesson 1: Cell Structures Involved In ...
-
Cell Transport And Homeostasis | CK-12 Foundation
-
The Cell Maintains Homeostasis. What Do You Mean By ... - Byju's
-
Homeostasis (article) | Feedback - Khan Academy
-
[DOC] The Cell Membrane
-
How Does The Cell Membrane Play A Role In Homeostasis?
-
[PDF] Homeostasis And Transport
-
Cellular Volume Homeostasis | Advances In Physiology Education
-
Mechanisms Orchestrating Mitochondrial Dynamics For Energy ...
-
Cell Size Control And Gene Expression Homeostasis In Single-cells