CO2 Lewis Structure, Molecular Geometry, Bond Angle, Polar Or ...
Maybe your like
CO2 Lewis structure is made up of one carbon (C) atom, and two oxygen (O) atoms. The carbon (C) atom is kept at the central position and the Oxygen (O) atom is on either side of it in the lewis diagram. In the CO2 lewis structure, there are a total of 4 lone pairs present.
A lewis structure helps us to know how electrons are arranged around individual atoms in a molecule.
Let’s see how to draw the lewis structure for CO2 with simple steps.
Follow some steps for drawing the Lewis dot structure for CO2
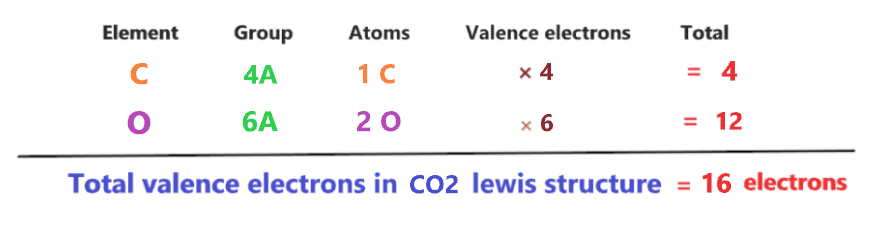
1. Count total valence electrons in CO2
As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. Valence electrons are the outermost electron of an atom that can participate in bond formation either by donating or accepting.
To find the total valence electron in CO2, look at the periodic group of carbon and oxygen atoms.
By looking at the periodic table, we come to know carbon belongs to 14 groups and oxygen belongs to the 16th group in the periodic table. Hence, carbon has 4 valence electrons and oxygen has 6 valence electrons.
⇒ Valence electron of Oxygen = 6 [∴ Periodic group of oxygen = 16 or 6A]
⇒ Valence electron of Carbon = 4 [∴ Periodic group of carbon = 14 or 4A]
∴ Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [∴ CO2 molecule has one carbon and two oxygen atoms]
2. Find the least electronegative atom and placed it at center
Now we need to find which atom(Carbon or oxygen) has the least electronegativity and then place that atom in the center of lewis’s diagram.
The electronegativity of the oxygen atom is 3.44 and for the carbon atom, it is 2.55
Clearly, the Carbon atom is less electronegative than Oxygen, therefore, place it at the center of the lewis diagram and put the oxygen atoms on either side of it.

3. Connect carbon and oxygen with a single bond
In the third step, we will start to draw the lewis structure of CO2 by connecting the outer atom (Oxygen) to the central atom (Carbon) with the help of a single bond.
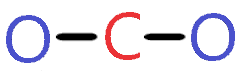
By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons. (A single bond means 2 electrons)
So, we used 4 electrons from a total of 16 valence electrons that are available for drawing the Lewis structure of CO2.
∴ (16 – 4) = 12 valence electrons
Now we are left with 12 valence electrons.
4. Placed remaining valence electrons around the outer atom
As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule.
“Octet rule show that atom is most stable when eight electrons present in its valence shell.”
So, oxygen needs 8 electrons around it for coming into the stable zone. Therefore, place the remaining valence electron around oxygen first for completing its octet shell.
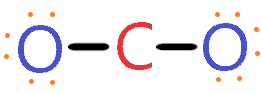
So, look at the above diagram and see how many valence electrons we used till now and how many are left. Each oxygen has 8 electrons(6 dot electrons + 2 electrons in a single bond), therefore, oxygen atoms completed their octets comfortably.
In the above diagram, 16 valence electrons are used(6 on each oxygen atom + 4 electrons in form of two single bonds).
So, we are left with zero valence electrons.
5. Complete the central atom octet and make a covalent bond if necessary
In this step, we have to complete the central atom(Carbon) octet for its stability.
As carbon needs 8 electrons to complete its octet shell but carbon has only 4 electrons(two single bonds) around it. (Look at the 4th step structure).
Therefore, the carbon atom needs 4 more electrons to complete its octet. Also, we have no extra valence electrons left for completing the octet of carbon.
So, to overcome this problem, we will take the help of oxygen lone pair electrons.
We will convert the one lone pair of each oxygen atom into a covalent bond as shown in the figure given below.
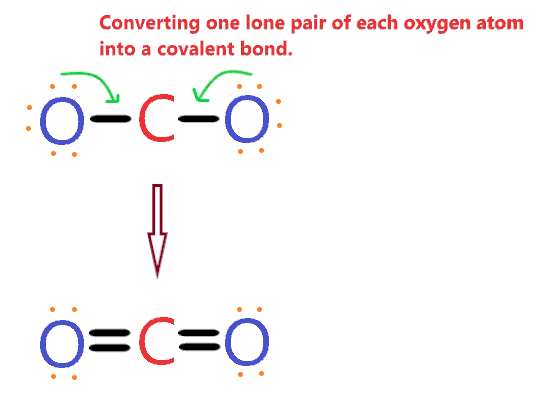
Now look at the above structure and see if the atoms of the CO2 molecule, completed their octet or not.
The carbon central atom has 8 electrons in its valence shell, since, it connected with 2 double bonds. [∴ 1 double bond means 4 electrons].
Also, both oxygen also has 8 electrons, as they are connected with one double bond means 4 electrons + 4 electrons represented as dots.
Yes, both atoms(Carbon and oxygen) have completed their octet rule comfortably as each of them has 8 electrons in the outermost shell.
Now just check the stability of the above structure with the help of the formal charge concept.
6. Check the stability of CO2 lewis structure with the help of a formal charge concept
“The lesser the formal charge on atoms, the better is the stability of the lewis structure.”
To calculate the formal charge on an atom. Use the formula given below-

We will calculate the formal charge for the 5th step structure.
For carbon atoms:
- Valence electrons of carbon = 4
- Nonbonding electrons on carbon = 0
- Bonding electrons around carbon (two double bonds) = 8
- ∴ (4 – 0 – 8/2) = 0 formal charge on the central carbon atom.
For oxygen atom
- Valence electrons of oxygen = 6
- Nonbonding electrons on oxygen = 4
- Bonding electrons around oxygen (one double bond) = 4
- ∴ (6 – 4 – 4/2) = 0 formal charge on the oxygen atom.
So, both atoms(carbon and oxygen) get a formal charge equal to zero.
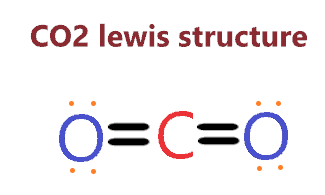
Carbon dioxide (CO2) lewis structure
Therefore, the above lewis structure of CO2 is better, appropriate, and most stable as the overall formal charge is zero.
Also check –
- Formal charge calculator
- Lewis structure generator
- How to draw a lewis structure?
Tag » Co2 Bond Shape
-
Some Molecules Have Multiple Bonds Which Affect Their VSEPR Shape.
-
CO2 Molecular Geometry And Bond Angles (Carbon Dioxide)
-
CO2 Lewis Structure, Molecular Geometry And Hybridization
-
CO2 Lewis Structure, Molecular Geometry, Molar Mass & Hybridization
-
Shape Of CO2 Is: | Chemistry Questions - Toppr
-
Shapes Of Molecules And Ions Containing Double Bonds - Chemguide
-
[PDF] SHAPES OF MOLECULES AND IONS (including Double Bonds)
-
CO2 Molecular Geometry - Science Education And Tutorials
-
Lewis Structure Of Carbon Dioxide - Byju's
-
[PDF] VSEPR Theory
-
Hybridization Of CO2 (Carbon Dioxide) - Byju's
-
What Is The Molecular Geometry Of Carbon Dioxide (CO2)? - Quora
-
CO2 Lewis Structure And Molecular Geometry - What's Insight