How Do You Find Core And Valence Electrons Class 11 Chemistry CBSE
Maybe your like
CoursesCourses for KidsFree study materialOffline CentresMore Store
Store

 Answer
Answer Question Answers for Class 12
Question Answers for Class 12 Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11
Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11 Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10
Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10 Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9
Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9 Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8
Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8 Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7
Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7 Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6
Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6 Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5
Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5 Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4
Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4 Class 4 ScienceClass 4 EnglishClass 4 Maths
Class 4 ScienceClass 4 EnglishClass 4 Maths
 How do you find core and valence electrons?Answer
How do you find core and valence electrons?Answer Verified548.4k+ viewsHint The answer to this question is based on the general concept of chemistry which includes the writing of the electronic configuration of the given atom and this gives the information about the core and the valence point.Complete answer:In the classes of chemistry, we have dealt with the topics which tell the basic composition and structure of an atom and also the properties of an atom.Now, we shall see how to identify the valence and core electrons by recalling the definition about the atom and also the relating terms of atoms.- An atom is composed of a central nucleus in which the positively charged protons and neutral neutrons are concentrated in and also the negatively charged species called the electrons are revolving around the orbit of the nucleus of an atom.- The core electrons are those electrons which are in the inner shell of an atom that are near to the nucleus and valence electrons are those which are the outermost electrons that are the electrons present in the outermost orbit.- Valence electrons participate in the bonding and core electrons do not participate in the bonding.If we consider an example of Carbon with an atomic number 6 and the atomic symbol$C$which has the electronic configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$, the core electrons is in the 1s orbital and there are 2 electrons and the valence electrons is in 2s and 2p orbital which are 4 in number.The diagram which depicts valence and core electrons is shown below,
Verified548.4k+ viewsHint The answer to this question is based on the general concept of chemistry which includes the writing of the electronic configuration of the given atom and this gives the information about the core and the valence point.Complete answer:In the classes of chemistry, we have dealt with the topics which tell the basic composition and structure of an atom and also the properties of an atom.Now, we shall see how to identify the valence and core electrons by recalling the definition about the atom and also the relating terms of atoms.- An atom is composed of a central nucleus in which the positively charged protons and neutral neutrons are concentrated in and also the negatively charged species called the electrons are revolving around the orbit of the nucleus of an atom.- The core electrons are those electrons which are in the inner shell of an atom that are near to the nucleus and valence electrons are those which are the outermost electrons that are the electrons present in the outermost orbit.- Valence electrons participate in the bonding and core electrons do not participate in the bonding.If we consider an example of Carbon with an atomic number 6 and the atomic symbol$C$which has the electronic configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$, the core electrons is in the 1s orbital and there are 2 electrons and the valence electrons is in 2s and 2p orbital which are 4 in number.The diagram which depicts valence and core electrons is shown below, 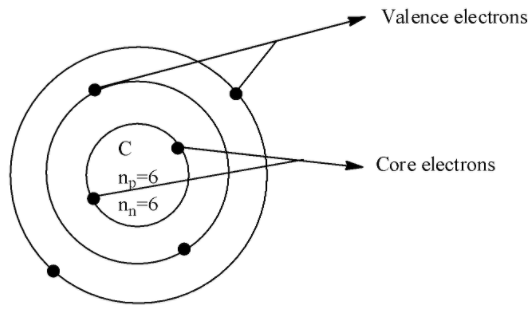 Thus, in this way we can find the core and valence electrons of the given atom.Note: Note that apart from core and valence electrons there is also lone pair of electrons which also participates in bonding but does not form the covalent bond and the lone pair of electrons can be understood by writing the Lewis structure.Recently Updated PagesWhy are manures considered better than fertilizers class 11 biology CBSE
Thus, in this way we can find the core and valence electrons of the given atom.Note: Note that apart from core and valence electrons there is also lone pair of electrons which also participates in bonding but does not form the covalent bond and the lone pair of electrons can be understood by writing the Lewis structure.Recently Updated PagesWhy are manures considered better than fertilizers class 11 biology CBSE Find the coordinates of the midpoint of the line segment class 11 maths CBSE
Find the coordinates of the midpoint of the line segment class 11 maths CBSE Distinguish between static friction limiting friction class 11 physics CBSE
Distinguish between static friction limiting friction class 11 physics CBSE The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE
The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE The first National Commission on Labour NCL submitted class 11 social science CBSE
The first National Commission on Labour NCL submitted class 11 social science CBSE Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE
Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE Why are manures considered better than fertilizers class 11 biology CBSE
Why are manures considered better than fertilizers class 11 biology CBSE Find the coordinates of the midpoint of the line segment class 11 maths CBSE
Find the coordinates of the midpoint of the line segment class 11 maths CBSE Distinguish between static friction limiting friction class 11 physics CBSE
Distinguish between static friction limiting friction class 11 physics CBSE The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE
The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE The first National Commission on Labour NCL submitted class 11 social science CBSE
The first National Commission on Labour NCL submitted class 11 social science CBSE Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE
Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE
 One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Difference Between Prokaryotic Cells and Eukaryotic Cells
Difference Between Prokaryotic Cells and Eukaryotic Cells 1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE
1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE State the laws of reflection of light
State the laws of reflection of light Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE 10 examples of friction in our daily life
10 examples of friction in our daily life One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Difference Between Prokaryotic Cells and Eukaryotic Cells
Difference Between Prokaryotic Cells and Eukaryotic Cells 1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE
1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE State the laws of reflection of light
State the laws of reflection of light Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE
Talk to our experts
1800-120-456-456
Sign In- Question Answer
- Class 11
- Chemistry
- How do you find core and valen...


 Question Answers for Class 12
Question Answers for Class 12
 How do you find core and valence electrons?Answer
How do you find core and valence electrons?Answer Thus, in this way we can find the core and valence electrons of the given atom.Note: Note that apart from core and valence electrons there is also lone pair of electrons which also participates in bonding but does not form the covalent bond and the lone pair of electrons can be understood by writing the Lewis structure.Recently Updated PagesWhy are manures considered better than fertilizers class 11 biology CBSE
Thus, in this way we can find the core and valence electrons of the given atom.Note: Note that apart from core and valence electrons there is also lone pair of electrons which also participates in bonding but does not form the covalent bond and the lone pair of electrons can be understood by writing the Lewis structure.Recently Updated PagesWhy are manures considered better than fertilizers class 11 biology CBSE Find the coordinates of the midpoint of the line segment class 11 maths CBSE
Find the coordinates of the midpoint of the line segment class 11 maths CBSE Distinguish between static friction limiting friction class 11 physics CBSE
Distinguish between static friction limiting friction class 11 physics CBSE The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE
The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE The first National Commission on Labour NCL submitted class 11 social science CBSE
The first National Commission on Labour NCL submitted class 11 social science CBSE Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE
Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE Why are manures considered better than fertilizers class 11 biology CBSE
Why are manures considered better than fertilizers class 11 biology CBSE Find the coordinates of the midpoint of the line segment class 11 maths CBSE
Find the coordinates of the midpoint of the line segment class 11 maths CBSE Distinguish between static friction limiting friction class 11 physics CBSE
Distinguish between static friction limiting friction class 11 physics CBSE The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE
The Chairman of the constituent Assembly was A Jawaharlal class 11 social science CBSE The first National Commission on Labour NCL submitted class 11 social science CBSE
The first National Commission on Labour NCL submitted class 11 social science CBSE Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE
Number of all subshell of n + l 7 is A 4 B 5 C 6 D class 11 chemistry CBSE
- 1
- 2
 One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Difference Between Prokaryotic Cells and Eukaryotic Cells
Difference Between Prokaryotic Cells and Eukaryotic Cells 1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE
1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE State the laws of reflection of light
State the laws of reflection of light Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE 10 examples of friction in our daily life
10 examples of friction in our daily life One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Difference Between Prokaryotic Cells and Eukaryotic Cells
Difference Between Prokaryotic Cells and Eukaryotic Cells 1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE
1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE State the laws of reflection of light
State the laws of reflection of light Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE
- 1
- 2
Repeaters Course for NEET 2022 - 23
NEET Repeater 2023 - Aakrosh 1 Year CourseTag » How To Find Core Electrons
-
How Do You Find Core And Valence Electrons? + Example - Socratic
-
Identify Valence Electrons And Core Electrons - YouTube
-
CHEMISTRY 101: Valence And Core Electrons - YouTube
-
Valence And Core Electrons - YouTube
-
How To Find Core Electrons And Valence Electrons By Using A Periodic ...
-
How To Find Core Electrons Of An Element? | Yeah Chemistry
-
1.9B: Valence And Core Electrons - Chemistry LibreTexts
-
Valence And Core Electrons - Energy Education
-
Core Electron - Wikipedia
-
2 - Core Electrons And Valence Electrons - Flux Science
-
Core Charge - Wikipedia
-
Give The Number Of Core Electrons For F^ - ? - Toppr
-
How Is Atomic Number Related To Core Electrons?