How Many Protons Do All Chlorine Atoms Have? - Vedantu
Maybe your like
CoursesCourses for KidsFree study materialOffline CentresMore Store
Store

 Answer
Answer Question Answers for Class 12
Question Answers for Class 12 Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11
Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11 Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10
Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10 Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9
Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9 Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8
Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8 Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7
Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7 Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6
Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6 Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5
Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5 Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4
Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4 Class 4 ScienceClass 4 EnglishClass 4 Maths
Class 4 ScienceClass 4 EnglishClass 4 Maths
 How many protons do all chlorine atoms have?Answer
How many protons do all chlorine atoms have?Answer Verified532.5k+ viewsHint: Number of protons in an element always remains the same and is equal to its atomic number. Also, number of proton = number of electron for the same atomComplete answer: It is very well known that chlorine is an element with the symbol Cl and atomic number 17. It belongs to the halogen family and appears between fluorine and bromine in the periodic table. Its properties are mostly intermediate between fluorine(F) and bromine(Br). It is a yellow-green gas at room temperature. Chlorine is an extremely reactive element and a strong oxidizing agent; also it has the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
Verified532.5k+ viewsHint: Number of protons in an element always remains the same and is equal to its atomic number. Also, number of proton = number of electron for the same atomComplete answer: It is very well known that chlorine is an element with the symbol Cl and atomic number 17. It belongs to the halogen family and appears between fluorine and bromine in the periodic table. Its properties are mostly intermediate between fluorine(F) and bromine(Br). It is a yellow-green gas at room temperature. Chlorine is an extremely reactive element and a strong oxidizing agent; also it has the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine. 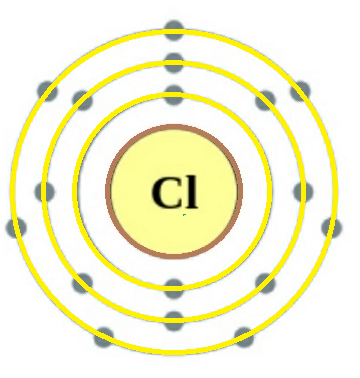 $_{17}^{35.45}Cl$ This is the atomic structure of the Chlorine atom and the way it is denoted is shown beside it. Here we can see that the atomic no. of Cl =17. Also no. of electrons of Cl atom shown in the structure is 17.As we know that, total no. of proton of an element = atomic no. of the elementSimilarly, total no. of electron in an atom = total no. of proton in an atom.By both ways we can conclude that:-Total no. of protons in all chlorine atom = 17 Additional Information: Total no. of neutrons can be calculated as $N=Z-A$where, A= atomic no. of the element Z= atomic mass of the elementNote: Chlorine will always have 17 protons no matter what isotope of chlorine is given, as an element's mass numbers can vary which means that it can have different numbers of neutrons.From this we conclude that, although chlorine has a mass number of 35 which means it has 18 neutrons and 17 protons, it can also have a mass number of 37, which means it has 20 neutrons and 17 protons.Recently Updated PagesMaster Class 10 Computer Science: Engaging Questions & Answers for Success
$_{17}^{35.45}Cl$ This is the atomic structure of the Chlorine atom and the way it is denoted is shown beside it. Here we can see that the atomic no. of Cl =17. Also no. of electrons of Cl atom shown in the structure is 17.As we know that, total no. of proton of an element = atomic no. of the elementSimilarly, total no. of electron in an atom = total no. of proton in an atom.By both ways we can conclude that:-Total no. of protons in all chlorine atom = 17 Additional Information: Total no. of neutrons can be calculated as $N=Z-A$where, A= atomic no. of the element Z= atomic mass of the elementNote: Chlorine will always have 17 protons no matter what isotope of chlorine is given, as an element's mass numbers can vary which means that it can have different numbers of neutrons.From this we conclude that, although chlorine has a mass number of 35 which means it has 18 neutrons and 17 protons, it can also have a mass number of 37, which means it has 20 neutrons and 17 protons.Recently Updated PagesMaster Class 10 Computer Science: Engaging Questions & Answers for Success Master Class 10 English: Engaging Questions & Answers for Success
Master Class 10 English: Engaging Questions & Answers for Success Master Class 10 Social Science: Engaging Questions & Answers for Success
Master Class 10 Social Science: Engaging Questions & Answers for Success Master Class 10 General Knowledge: Engaging Questions & Answers for Success
Master Class 10 General Knowledge: Engaging Questions & Answers for Success Master Class 10 Maths: Engaging Questions & Answers for Success
Master Class 10 Maths: Engaging Questions & Answers for Success Master Class 10 Science: Engaging Questions & Answers for Success
Master Class 10 Science: Engaging Questions & Answers for Success Master Class 10 Computer Science: Engaging Questions & Answers for Success
Master Class 10 Computer Science: Engaging Questions & Answers for Success Master Class 10 English: Engaging Questions & Answers for Success
Master Class 10 English: Engaging Questions & Answers for Success Master Class 10 Social Science: Engaging Questions & Answers for Success
Master Class 10 Social Science: Engaging Questions & Answers for Success Master Class 10 General Knowledge: Engaging Questions & Answers for Success
Master Class 10 General Knowledge: Engaging Questions & Answers for Success Master Class 10 Maths: Engaging Questions & Answers for Success
Master Class 10 Maths: Engaging Questions & Answers for Success Master Class 10 Science: Engaging Questions & Answers for Success
Master Class 10 Science: Engaging Questions & Answers for Success
 Who Won 36 Oscar Awards? Record Holder Revealed
Who Won 36 Oscar Awards? Record Holder Revealed What is the median of the first 10 natural numbers class 10 maths CBSE
What is the median of the first 10 natural numbers class 10 maths CBSE Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE
Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE Write a letter to the principal requesting him to grant class 10 english CBSE
Write a letter to the principal requesting him to grant class 10 english CBSE Who is the executive head of the government APresident class 10 social science CBSE
Who is the executive head of the government APresident class 10 social science CBSE Why is there a time difference of about 5 hours between class 10 social science CBSE
Why is there a time difference of about 5 hours between class 10 social science CBSE Who Won 36 Oscar Awards? Record Holder Revealed
Who Won 36 Oscar Awards? Record Holder Revealed What is the median of the first 10 natural numbers class 10 maths CBSE
What is the median of the first 10 natural numbers class 10 maths CBSE Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE
Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE Write a letter to the principal requesting him to grant class 10 english CBSE
Write a letter to the principal requesting him to grant class 10 english CBSE Who is the executive head of the government APresident class 10 social science CBSE
Who is the executive head of the government APresident class 10 social science CBSE
Talk to our experts
1800-120-456-456
Sign In- Question Answer
- Class 10
- Science
- How many protons do all chlori...


 Question Answers for Class 12
Question Answers for Class 12
 How many protons do all chlorine atoms have?Answer
How many protons do all chlorine atoms have?Answer $_{17}^{35.45}Cl$ This is the atomic structure of the Chlorine atom and the way it is denoted is shown beside it. Here we can see that the atomic no. of Cl =17. Also no. of electrons of Cl atom shown in the structure is 17.As we know that, total no. of proton of an element = atomic no. of the elementSimilarly, total no. of electron in an atom = total no. of proton in an atom.By both ways we can conclude that:-Total no. of protons in all chlorine atom = 17 Additional Information: Total no. of neutrons can be calculated as $N=Z-A$where, A= atomic no. of the element Z= atomic mass of the elementNote: Chlorine will always have 17 protons no matter what isotope of chlorine is given, as an element's mass numbers can vary which means that it can have different numbers of neutrons.From this we conclude that, although chlorine has a mass number of 35 which means it has 18 neutrons and 17 protons, it can also have a mass number of 37, which means it has 20 neutrons and 17 protons.Recently Updated PagesMaster Class 10 Computer Science: Engaging Questions & Answers for Success
$_{17}^{35.45}Cl$ This is the atomic structure of the Chlorine atom and the way it is denoted is shown beside it. Here we can see that the atomic no. of Cl =17. Also no. of electrons of Cl atom shown in the structure is 17.As we know that, total no. of proton of an element = atomic no. of the elementSimilarly, total no. of electron in an atom = total no. of proton in an atom.By both ways we can conclude that:-Total no. of protons in all chlorine atom = 17 Additional Information: Total no. of neutrons can be calculated as $N=Z-A$where, A= atomic no. of the element Z= atomic mass of the elementNote: Chlorine will always have 17 protons no matter what isotope of chlorine is given, as an element's mass numbers can vary which means that it can have different numbers of neutrons.From this we conclude that, although chlorine has a mass number of 35 which means it has 18 neutrons and 17 protons, it can also have a mass number of 37, which means it has 20 neutrons and 17 protons.Recently Updated PagesMaster Class 10 Computer Science: Engaging Questions & Answers for Success Master Class 10 English: Engaging Questions & Answers for Success
Master Class 10 English: Engaging Questions & Answers for Success Master Class 10 Social Science: Engaging Questions & Answers for Success
Master Class 10 Social Science: Engaging Questions & Answers for Success Master Class 10 General Knowledge: Engaging Questions & Answers for Success
Master Class 10 General Knowledge: Engaging Questions & Answers for Success Master Class 10 Maths: Engaging Questions & Answers for Success
Master Class 10 Maths: Engaging Questions & Answers for Success Master Class 10 Science: Engaging Questions & Answers for Success
Master Class 10 Science: Engaging Questions & Answers for Success Master Class 10 Computer Science: Engaging Questions & Answers for Success
Master Class 10 Computer Science: Engaging Questions & Answers for Success Master Class 10 English: Engaging Questions & Answers for Success
Master Class 10 English: Engaging Questions & Answers for Success Master Class 10 Social Science: Engaging Questions & Answers for Success
Master Class 10 Social Science: Engaging Questions & Answers for Success Master Class 10 General Knowledge: Engaging Questions & Answers for Success
Master Class 10 General Knowledge: Engaging Questions & Answers for Success Master Class 10 Maths: Engaging Questions & Answers for Success
Master Class 10 Maths: Engaging Questions & Answers for Success Master Class 10 Science: Engaging Questions & Answers for Success
Master Class 10 Science: Engaging Questions & Answers for Success
- 1
- 2
 Who Won 36 Oscar Awards? Record Holder Revealed
Who Won 36 Oscar Awards? Record Holder Revealed What is the median of the first 10 natural numbers class 10 maths CBSE
What is the median of the first 10 natural numbers class 10 maths CBSE Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE
Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE Write a letter to the principal requesting him to grant class 10 english CBSE
Write a letter to the principal requesting him to grant class 10 english CBSE Who is the executive head of the government APresident class 10 social science CBSE
Who is the executive head of the government APresident class 10 social science CBSE Why is there a time difference of about 5 hours between class 10 social science CBSE
Why is there a time difference of about 5 hours between class 10 social science CBSE Who Won 36 Oscar Awards? Record Holder Revealed
Who Won 36 Oscar Awards? Record Holder Revealed What is the median of the first 10 natural numbers class 10 maths CBSE
What is the median of the first 10 natural numbers class 10 maths CBSE Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE
Who was Subhash Chandra Bose Why was he called Net class 10 english CBSE Write a letter to the principal requesting him to grant class 10 english CBSE
Write a letter to the principal requesting him to grant class 10 english CBSE Who is the executive head of the government APresident class 10 social science CBSE
Who is the executive head of the government APresident class 10 social science CBSE
- 1
- 2
Tag » How Many Protons Are In Chlorine
-
Chlorine Facts - Chlorine The Element Of Surprise
-
How Many Electrons, Protons, And Neutrons Does Chlorine Have?
-
How To Find The Number Of Protons, Electrons, Neutrons For Chlorine ...
-
How Many Protons, Neutrons, And Electrons Are In Chlorine-35 ...
-
1.2: Isotopes - Chemistry LibreTexts
-
Structure Of The Nucleus - GCSE Physics (Single Science) Revision
-
Chlorine - Element Information, Properties And Uses | Periodic Table
-
[PDF] Atomic Numbers - Atomic Masses
-
How Many Protons Are In The Nucleus Of An Atom Of Chlorine? | Socratic
-
How Many Protons Do All Chlorine Atoms Have? - Toppr
-
How Many Neutrons Are In An Atom Of Chlorine? - Toppr
-
Questions And Answers - Why Aren't Chlorine-35 And Chlorine-37 ...
-
Chlorine-37 - Wikipedia
-
For Example, Every Chlorine Atom Has 17 Electrons And 17 Protons