Introduction To Buffers - Chemistry LibreTexts
Maybe your like
How does a buffer work?
A buffer is able to resist pH change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts at equilibrium and are able to neutralize small amounts of other acids and bases (in the form of H3O+ and OH-) when the are added to the solution. To clarify this effect, we can consider the simple example of a Hydrofluoric Acid (HF) and Sodium Fluoride (NaF) buffer. Hydrofluoric acid is a weak acid due to the strong attraction between the relatively small F- ion and solvated protons (H3O+), which does not allow it to dissociate completely in water. Therefore, if we obtain HF in an aqueous solution, we establish the following equilibrium with only slight dissociation (Ka(HF) = 6.6x10-4, strongly favors reactants):
\[HF_{(aq)} + H_2O_{(l)} \rightleftharpoons F^-_{(aq)} + H_3O^+_{(aq)} \nonumber \]
We can then add and dissolve sodium fluoride into the solution and mix the two until we reach the desired volume and pH at which we want to buffer. When Sodium Fluoride dissolves in water, the reaction goes to completion, thus we obtain:
\[NaF_{(aq)} + H_2O_{(l)} \rightarrow Na^+_{(aq)} + F^-_{(aq)} \nonumber \]
Since Na+ is the conjugate of a strong base, it will have no effect on the pH or reactivity of the buffer. The addition of \(NaF\) to the solution will, however, increase the concentration of F- in the buffer solution, and, consequently, by Le Chatelier’s Principle, lead to slightly less dissociation of the HF in the previous equilibrium, as well. The presence of significant amounts of both the conjugate acid, \(HF\), and the conjugate base, F-, allows the solution to function as a buffer. This buffering action can be seen in the titration curve of a buffer solution.
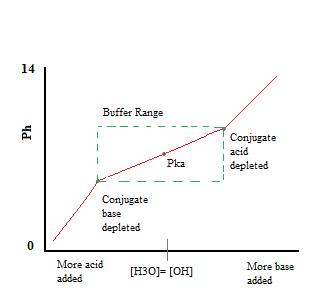
As we can see, over the working range of the buffer. pH changes very little with the addition of acid or base. Once the buffering capacity is exceeded the rate of pH change quickly jumps. This occurs because the conjugate acid or base has been depleted through neutralization. This principle implies that a larger amount of conjugate acid or base will have a greater buffering capacity.
If acid were added:
\[F^-_{(aq)} + H_3O^+_{(aq)} \rightleftharpoons HF_{(aq)} + H_2O_{(l)} \nonumber \]
In this reaction, the conjugate base, F-, will neutralize the added acid, H3O+, and this reaction goes to completion, because the reaction of F- with H3O+ has an equilibrium constant much greater than one. (In fact, the equilibrium constant the reaction as written is just the inverse of the Ka for HF: 1/Ka(HF) = 1/(6.6x10-4) = 1.5x10+3.) So long as there is more F- than H3O+, almost all of the H3O+ will be consumed and the equilibrium will shift to the right, slightly increasing the concentration of HF and slightly decreasing the concentration of F-, but resulting in hardly any change in the amount of H3O+ present once equilibrium is re-established.
If base were added:
\[HF_{(aq)} + OH^-_{(aq)} \rightleftharpoons F^-_{(aq)} + H_2O_{(l)} \nonumber \]
In this reaction, the conjugate acid, HF, will neutralize added amounts of base, OH-, and the equilibrium will again shift to the right, slightly increasing the concentration of F- in the solution and decreasing the amount of HF slightly. Again, since most of the OH- is neutralized, little pH change will occur.
These two reactions can continue to alternate back and forth with little pH change.
Tag » How To Determine A Buffer Solution
-
Calculation Of The PH Of A Buffer Solution
-
Buffer Solutions (video) | Khan Academy
-
Tips For IDing If A Solution Is A Buffer - Chemistry - Brightstorm
-
Buffer Solutions - Chemguide
-
Determine-acidic-and-alkaline-buffer-solution - Chemistry Guru
-
Calculate-ph-of-buffer-solution - Chemistry Guru
-
6.8: Buffer Solutions - Chemistry LibreTexts
-
Buffer Solutions - YouTube
-
How Can We Identify Buffer Solution, Acidic Buffer And Basic ... - Quora
-
Predicting The PH Of A Buffer - ChemCollective
-
Buffer PH Calculator
-
How To Determine Acidic And Alkaline Buffer Solutions - Ionic Equilibria
-
Buffer Solution - Acidic And Basic Buffers, Preparations, Examples
-
How To Calculate PH Of Buffer Solutions - Sciencing