Ions When An Ionic Compound Is Dissolved In Water Can Class 11 ...
Maybe your like
CoursesCourses for KidsFree study materialOffline CentresMore Store
Store

 Answer
Answer Question Answers for Class 12
Question Answers for Class 12 Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11
Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11 Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10
Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10 Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9
Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9 Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8
Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8 Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7
Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7 Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6
Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6 Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5
Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5 Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4
Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4 Class 4 ScienceClass 4 EnglishClass 4 Maths
Class 4 ScienceClass 4 EnglishClass 4 Maths
 Ions, when an ionic compound is dissolved in water, can best be described as:A. Hydrated molecules onlyB. Dehydrated ions and moleculesC. Both hydrated molecules and hydrated ionsD. Neither hydrated ions nor hydrated moleculesE. Hydrated ions onlyAnswer
Ions, when an ionic compound is dissolved in water, can best be described as:A. Hydrated molecules onlyB. Dehydrated ions and moleculesC. Both hydrated molecules and hydrated ionsD. Neither hydrated ions nor hydrated moleculesE. Hydrated ions onlyAnswer Verified584.7k+ viewsHint: We know that the ionic compounds are those compounds that are usually made up of ions and give both the charges that are positive and negative.Complete step by step answer:As we know that, the ionic compounds are dissolved in water for the reason that the water molecules hydrate their ions. When the ionic compounds dissolve in water, the water molecules stabilize the ions that are generally caused by breaking the ionic bond. They only do this for hydrating the produced ions.The water is one of the polar molecules. It usually has the permanent dipole moment. The oxygen atom has a partial negative charge, and the hydrogen atom has a partial positive charge. When the ionic constituent is placed in water, then the water molecules appeal to the positive and negative ions of the crystal. The positive ions are covered with oxygen atoms, and they have several water molecules of a positive charge.Similarly, the negative ions are covered with hydrogen atoms, and they have several water molecules of the negative charge. The water molecules reduce the attractions between the ionic ions. So, the ions are hydrated. The diagram of hydrate ions is given below:
Verified584.7k+ viewsHint: We know that the ionic compounds are those compounds that are usually made up of ions and give both the charges that are positive and negative.Complete step by step answer:As we know that, the ionic compounds are dissolved in water for the reason that the water molecules hydrate their ions. When the ionic compounds dissolve in water, the water molecules stabilize the ions that are generally caused by breaking the ionic bond. They only do this for hydrating the produced ions.The water is one of the polar molecules. It usually has the permanent dipole moment. The oxygen atom has a partial negative charge, and the hydrogen atom has a partial positive charge. When the ionic constituent is placed in water, then the water molecules appeal to the positive and negative ions of the crystal. The positive ions are covered with oxygen atoms, and they have several water molecules of a positive charge.Similarly, the negative ions are covered with hydrogen atoms, and they have several water molecules of the negative charge. The water molecules reduce the attractions between the ionic ions. So, the ions are hydrated. The diagram of hydrate ions is given below: 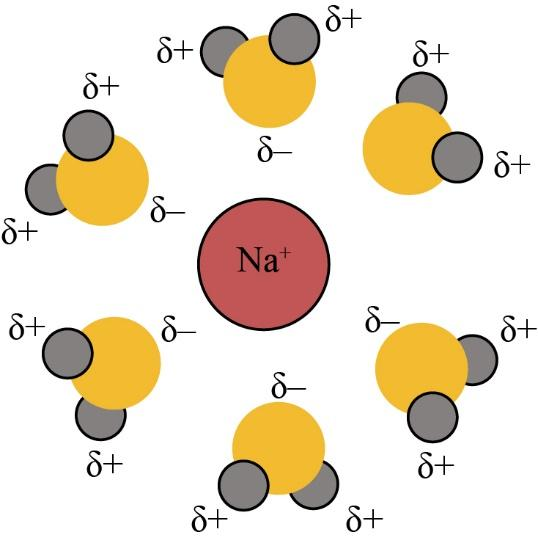 So, the correct answer is “Option E”.Note:The ionic compounds are dissolved in water then their ions are hydrated in it. The ionic compounds are water-soluble.Recently Updated PagesMaster Class 11 Computer Science: Engaging Questions & Answers for Success
So, the correct answer is “Option E”.Note:The ionic compounds are dissolved in water then their ions are hydrated in it. The ionic compounds are water-soluble.Recently Updated PagesMaster Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Maths: Engaging Questions & Answers for Success
Master Class 11 Maths: Engaging Questions & Answers for Success Master Class 11 Biology: Engaging Questions & Answers for Success
Master Class 11 Biology: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Maths: Engaging Questions & Answers for Success
Master Class 11 Maths: Engaging Questions & Answers for Success Master Class 11 Biology: Engaging Questions & Answers for Success
Master Class 11 Biology: Engaging Questions & Answers for Success
 One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE State the laws of reflection of light
State the laws of reflection of light 10 examples of friction in our daily life
10 examples of friction in our daily life Who is known as the father of chemistry class 11 chemistry CBSE
Who is known as the father of chemistry class 11 chemistry CBSE There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE State the laws of reflection of light
State the laws of reflection of light 10 examples of friction in our daily life
10 examples of friction in our daily life Who is known as the father of chemistry class 11 chemistry CBSE
Who is known as the father of chemistry class 11 chemistry CBSE
Talk to our experts
1800-120-456-456
Sign In- Question Answer
- Class 11
- Chemistry
- Ions when an ionic compound is...


 Question Answers for Class 12
Question Answers for Class 12
 Ions, when an ionic compound is dissolved in water, can best be described as:A. Hydrated molecules onlyB. Dehydrated ions and moleculesC. Both hydrated molecules and hydrated ionsD. Neither hydrated ions nor hydrated moleculesE. Hydrated ions onlyAnswer
Ions, when an ionic compound is dissolved in water, can best be described as:A. Hydrated molecules onlyB. Dehydrated ions and moleculesC. Both hydrated molecules and hydrated ionsD. Neither hydrated ions nor hydrated moleculesE. Hydrated ions onlyAnswer So, the correct answer is “Option E”.Note:The ionic compounds are dissolved in water then their ions are hydrated in it. The ionic compounds are water-soluble.Recently Updated PagesMaster Class 11 Computer Science: Engaging Questions & Answers for Success
So, the correct answer is “Option E”.Note:The ionic compounds are dissolved in water then their ions are hydrated in it. The ionic compounds are water-soluble.Recently Updated PagesMaster Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Maths: Engaging Questions & Answers for Success
Master Class 11 Maths: Engaging Questions & Answers for Success Master Class 11 Biology: Engaging Questions & Answers for Success
Master Class 11 Biology: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Maths: Engaging Questions & Answers for Success
Master Class 11 Maths: Engaging Questions & Answers for Success Master Class 11 Biology: Engaging Questions & Answers for Success
Master Class 11 Biology: Engaging Questions & Answers for Success
- 1
- 2
 One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE State the laws of reflection of light
State the laws of reflection of light 10 examples of friction in our daily life
10 examples of friction in our daily life Who is known as the father of chemistry class 11 chemistry CBSE
Who is known as the father of chemistry class 11 chemistry CBSE There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE Explain zero factorial class 11 maths CBSE
Explain zero factorial class 11 maths CBSE State the laws of reflection of light
State the laws of reflection of light 10 examples of friction in our daily life
10 examples of friction in our daily life Who is known as the father of chemistry class 11 chemistry CBSE
Who is known as the father of chemistry class 11 chemistry CBSE
- 1
- 2
Repeaters Course for NEET 2022 - 23
NEET Repeater 2023 - Aakrosh 1 Year CourseTag » What Happens When An Ionic Compound Dissolves In Water
-
What Happens To Ionic & Covalent Compounds When They Dissolve ...
-
Aqueous Solutions And Solubility - Compounds Dissolved In Water
-
What Happens When An Ionic Compound Dissolves In ... - Sciencing
-
What Happens When An Ionic Compound Dissolves? - Byju's
-
What Happens When Ionic Compounds Dissolve In Water? - Quora
-
Why Do Ionic Compounds Dissolve In Water? - Socratic
-
Are Ionic Compounds Soluble In Water? | O Level Chemistry Notes
-
Dissolving Ionic Compounds In Water - YouTube
-
Solubility And Complex-Ion Equilibria
-
Why Do Ionic Compounds Dissolve In Water? - Toppr
-
Solved What Happens When Ionic Compounds Dissolve In Water?
-
11.2 Electrolytes – Chemistry - ECampusOntario Pressbooks
-
Do All Ionic Compounds Dissolve In Water? - Benjamin-Mills
-
What Happens When An Ionic Compound Dissolves In Water?