Methane With The Molecular Formula “CH4” Has A. 4 Covalent Bonds ...
Maybe your like
CoursesCourses for KidsFree study materialOffline CentresMore Store
Store

 Answer
Answer Question Answers for Class 12
Question Answers for Class 12 Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11
Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11 Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10
Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10 Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9
Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9 Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8
Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8 Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7
Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7 Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6
Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6 Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5
Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5 Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4
Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4 Class 4 ScienceClass 4 EnglishClass 4 Maths
Class 4 ScienceClass 4 EnglishClass 4 Maths
 Methane with the Molecular formula “$C{H_4}$” hasA. 4 covalent bondsB. 8 covalent bondsC. 6 Covalent bondsD. 2 Covalent bondsAnswer
Methane with the Molecular formula “$C{H_4}$” hasA. 4 covalent bondsB. 8 covalent bondsC. 6 Covalent bondsD. 2 Covalent bondsAnswer Verified578.1k+ viewsHint: Carbon has four valence electrons in the outermost shell that’s why it can share its four electrons only. The covalent bond is formed when the atoms share their valence electrons with each other to form the chemical bond.Complete step by step answer: Methane is the simplest hydrocarbon with molecular formula $C{H_4}$.The atomic number of carbon is 6. The electronic configuration of carbon is $[He]2{s^2}2{p^2}$. Total four electrons are present in the valence shell of the carbon. The carbon can either lose its four electrons or gain its four electrons to obtain its stability by achieving eight electrons but due to high energy requirements the carbon cannot lose or gain electrons to form ions. So it forms a covalent bond with the atoms by sharing its four electrons with the four other atoms.The covalent bond is formed by the sharing of electrons between the atoms to form the compound.In methane the four electrons of carbon bind with the four electrons of four hydrogen atoms to form four covalent bonds. In methane carbon atoms are bonded with four hydrogen atoms.The structure of the methane is shown below.
Verified578.1k+ viewsHint: Carbon has four valence electrons in the outermost shell that’s why it can share its four electrons only. The covalent bond is formed when the atoms share their valence electrons with each other to form the chemical bond.Complete step by step answer: Methane is the simplest hydrocarbon with molecular formula $C{H_4}$.The atomic number of carbon is 6. The electronic configuration of carbon is $[He]2{s^2}2{p^2}$. Total four electrons are present in the valence shell of the carbon. The carbon can either lose its four electrons or gain its four electrons to obtain its stability by achieving eight electrons but due to high energy requirements the carbon cannot lose or gain electrons to form ions. So it forms a covalent bond with the atoms by sharing its four electrons with the four other atoms.The covalent bond is formed by the sharing of electrons between the atoms to form the compound.In methane the four electrons of carbon bind with the four electrons of four hydrogen atoms to form four covalent bonds. In methane carbon atoms are bonded with four hydrogen atoms.The structure of the methane is shown below. 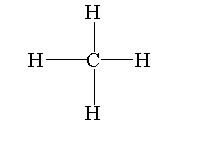 Thus, methane with the molecular formula $C{H_4}$ has 4 covalent bonds.So, the correct answer is “Option A”.Note:Carbon is a tetravalent compound therefore, it can only form four covalent bonds with other atoms and no more covalent bond is formed. The carbon compound can form carbocation and carbanion by losing and gaining one electron.Recently Updated PagesBasicity of sulphurous acid and sulphuric acid are
Thus, methane with the molecular formula $C{H_4}$ has 4 covalent bonds.So, the correct answer is “Option A”.Note:Carbon is a tetravalent compound therefore, it can only form four covalent bonds with other atoms and no more covalent bond is formed. The carbon compound can form carbocation and carbanion by losing and gaining one electron.Recently Updated PagesBasicity of sulphurous acid and sulphuric acid are Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Basicity of sulphurous acid and sulphuric acid are
Basicity of sulphurous acid and sulphuric acid are Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success
 State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE
Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE The transition element that has lowest enthalpy of class 11 chemistry CBSE
The transition element that has lowest enthalpy of class 11 chemistry CBSE Can anyone list 10 advantages and disadvantages of friction
Can anyone list 10 advantages and disadvantages of friction State the laws of reflection of light
State the laws of reflection of light One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE
Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE The transition element that has lowest enthalpy of class 11 chemistry CBSE
The transition element that has lowest enthalpy of class 11 chemistry CBSE Can anyone list 10 advantages and disadvantages of friction
Can anyone list 10 advantages and disadvantages of friction State the laws of reflection of light
State the laws of reflection of light
Talk to our experts
1800-120-456-456
Sign In- Question Answer
- Class 11
- Chemistry
- Methane with the Molecular for...


 Question Answers for Class 12
Question Answers for Class 12
 Methane with the Molecular formula “$C{H_4}$” hasA. 4 covalent bondsB. 8 covalent bondsC. 6 Covalent bondsD. 2 Covalent bondsAnswer
Methane with the Molecular formula “$C{H_4}$” hasA. 4 covalent bondsB. 8 covalent bondsC. 6 Covalent bondsD. 2 Covalent bondsAnswer Thus, methane with the molecular formula $C{H_4}$ has 4 covalent bonds.So, the correct answer is “Option A”.Note:Carbon is a tetravalent compound therefore, it can only form four covalent bonds with other atoms and no more covalent bond is formed. The carbon compound can form carbocation and carbanion by losing and gaining one electron.Recently Updated PagesBasicity of sulphurous acid and sulphuric acid are
Thus, methane with the molecular formula $C{H_4}$ has 4 covalent bonds.So, the correct answer is “Option A”.Note:Carbon is a tetravalent compound therefore, it can only form four covalent bonds with other atoms and no more covalent bond is formed. The carbon compound can form carbocation and carbanion by losing and gaining one electron.Recently Updated PagesBasicity of sulphurous acid and sulphuric acid are Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Basicity of sulphurous acid and sulphuric acid are
Basicity of sulphurous acid and sulphuric acid are Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success
- 1
- 2
 State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE
Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE The transition element that has lowest enthalpy of class 11 chemistry CBSE
The transition element that has lowest enthalpy of class 11 chemistry CBSE Can anyone list 10 advantages and disadvantages of friction
Can anyone list 10 advantages and disadvantages of friction State the laws of reflection of light
State the laws of reflection of light One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE
Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE The transition element that has lowest enthalpy of class 11 chemistry CBSE
The transition element that has lowest enthalpy of class 11 chemistry CBSE Can anyone list 10 advantages and disadvantages of friction
Can anyone list 10 advantages and disadvantages of friction State the laws of reflection of light
State the laws of reflection of light
- 1
- 2
Repeaters Course for NEET 2022 - 23
NEET Repeater 2023 - Aakrosh 1 Year CourseTag » What Kind Of Bond Is Ch4
-
Is Methane A Single Or Double Bond? - Byju's
-
Type Of Bonds For CH4 (Methane) - YouTube
-
Is CH4 Ionic Or Covalent? - Techiescientist
-
Chemical Bonds And Attractive Forces - Chemistry Tutorial
-
The Bonds Present In CH4 Are : | Chemistry Questions - Toppr
-
Ionic Vs Covalent – Easy Hard Science - Learn With Dr. Scott
-
What Type Of Bond Is Present In Methane? - Quora
-
Is CH4 An Ionic Or Covalent Bond?
-
Bonding In Methane - Chemistry LibreTexts
-
Types Of Covalent Bonds: Polar And Nonpolar
-
Methane | CH4 - PubChem
-
Molecular Structure & Bonding - MSU Chemistry
-
What Type Of Bonds Are Present In Methane (CH4) And Solution ...
-
What Type Of Bonds Are Present In Methane CH4 And Sodium ...