What Happened When: BF3 Is Reacted With Ammonia? - Vedantu
Maybe your like
CoursesCourses for KidsFree study materialOffline CentresMore Store
Store

 Answer
Answer Question Answers for Class 12
Question Answers for Class 12 Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11
Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11 Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10
Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10 Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9
Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9 Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8
Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8 Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7
Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7 Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6
Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6 Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5
Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5 Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4
Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4 Class 4 ScienceClass 4 EnglishClass 4 Maths
Class 4 ScienceClass 4 EnglishClass 4 Maths
 What happened when:$B{{F}_{3}}$ is reacted with ammonia?Answer
What happened when:$B{{F}_{3}}$ is reacted with ammonia?Answer Verified572.7k+ viewsHint: The $B{{F}_{3}}$ is a Lewis acid i.e. an electron deficient species and $N{{H}_{3}}$ is a Lewis base, a species which consists of lone pairs that can be donated for the formation of bonds.- $N{{H}_{3}}$ molecule consists of a lone pair of electrons .Complete Solution :So here in the question we are asked to predict what happens when $N{{H}_{3}}$ and $B{{F}_{3}}$ reacts together.Before predicting the product let’s see some characteristics of ammonia and boron trifluoride.So in $B{{F}_{3}}$ molecule B is the central atom and we know that the$B{{F}_{3}}$ is a Lewis acid i.e. an electron deficient species.- The atomic number of B is 5 and has an electronic configuration:$\text{E}\text{.C}\,\text{of}\,\text{B=1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{1}}}$- The valency possessed by the B atom is +3.- The hybridization of $B{{F}_{3}}$ molecule is $s{{p}^{2}}$ and hence having a planar structure.- In $N{{H}_{3}}$ molecule, N is the central atom having the atomic number 7 and the electronic configuration is,$\text{E}\text{.C}\,\text{of}\,\text{N=1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{3}}}$Ammonia is a $s{{p}^{3}}$ hybridized molecule in which one lone pair is present and hence having a trigonal pyramidal structure.So as we discussed earlier $B{{F}_{3}}$ is a Lewis acid and requires two electrons for B to obtain the octet configuration and $N{{H}_{3}}$ consists of one lone pair of electron and it acts as the Lewis base according to Lewis theory.Hence the $N{{H}_{3}}$ molecule donates its lone pair of electrons to the $B{{F}_{3}}$ molecule and forms a coordinate bond.So the ammonia and boron trifluoride on reactions forms an adduct.
Verified572.7k+ viewsHint: The $B{{F}_{3}}$ is a Lewis acid i.e. an electron deficient species and $N{{H}_{3}}$ is a Lewis base, a species which consists of lone pairs that can be donated for the formation of bonds.- $N{{H}_{3}}$ molecule consists of a lone pair of electrons .Complete Solution :So here in the question we are asked to predict what happens when $N{{H}_{3}}$ and $B{{F}_{3}}$ reacts together.Before predicting the product let’s see some characteristics of ammonia and boron trifluoride.So in $B{{F}_{3}}$ molecule B is the central atom and we know that the$B{{F}_{3}}$ is a Lewis acid i.e. an electron deficient species.- The atomic number of B is 5 and has an electronic configuration:$\text{E}\text{.C}\,\text{of}\,\text{B=1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{1}}}$- The valency possessed by the B atom is +3.- The hybridization of $B{{F}_{3}}$ molecule is $s{{p}^{2}}$ and hence having a planar structure.- In $N{{H}_{3}}$ molecule, N is the central atom having the atomic number 7 and the electronic configuration is,$\text{E}\text{.C}\,\text{of}\,\text{N=1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{3}}}$Ammonia is a $s{{p}^{3}}$ hybridized molecule in which one lone pair is present and hence having a trigonal pyramidal structure.So as we discussed earlier $B{{F}_{3}}$ is a Lewis acid and requires two electrons for B to obtain the octet configuration and $N{{H}_{3}}$ consists of one lone pair of electron and it acts as the Lewis base according to Lewis theory.Hence the $N{{H}_{3}}$ molecule donates its lone pair of electrons to the $B{{F}_{3}}$ molecule and forms a coordinate bond.So the ammonia and boron trifluoride on reactions forms an adduct. 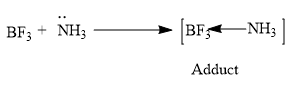 $N{{H}_{3}}$ The hybridization of this adduct formed is $s{{p}^{3}}$ hybridized adduct.Note: The hybridization of a molecule is calculate using the formulae,$\text{steric number = No}\text{. of bps + No}\text{.of lps}$Bps refers to bond pairs and lps refers to lone pairs and the steric number obtained will give an idea about the hybridization of the molecule.Recently Updated PagesMaster Class 11 Business Studies: Engaging Questions & Answers for Success
$N{{H}_{3}}$ The hybridization of this adduct formed is $s{{p}^{3}}$ hybridized adduct.Note: The hybridization of a molecule is calculate using the formulae,$\text{steric number = No}\text{. of bps + No}\text{.of lps}$Bps refers to bond pairs and lps refers to lone pairs and the steric number obtained will give an idea about the hybridization of the molecule.Recently Updated PagesMaster Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Chemistry: Engaging Questions & Answers for Success
Master Class 11 Chemistry: Engaging Questions & Answers for Success Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Chemistry: Engaging Questions & Answers for Success
Master Class 11 Chemistry: Engaging Questions & Answers for Success
 The period of a conical pendulum in terms of its length class 11 physics CBSE
The period of a conical pendulum in terms of its length class 11 physics CBSE One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE
In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE What is the difference between free and forced vib class 11 physics CBSE
What is the difference between free and forced vib class 11 physics CBSE A large number of liquid drops each of radius r coalesce class 11 physics CBSE
A large number of liquid drops each of radius r coalesce class 11 physics CBSE The period of a conical pendulum in terms of its length class 11 physics CBSE
The period of a conical pendulum in terms of its length class 11 physics CBSE One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE
In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE What is the difference between free and forced vib class 11 physics CBSE
What is the difference between free and forced vib class 11 physics CBSE
Talk to our experts
1800-120-456-456
Sign In- Question Answer
- Class 11
- Chemistry
- What happened when BF3 is reac...


 Question Answers for Class 12
Question Answers for Class 12
 What happened when:$B{{F}_{3}}$ is reacted with ammonia?Answer
What happened when:$B{{F}_{3}}$ is reacted with ammonia?Answer $N{{H}_{3}}$ The hybridization of this adduct formed is $s{{p}^{3}}$ hybridized adduct.Note: The hybridization of a molecule is calculate using the formulae,$\text{steric number = No}\text{. of bps + No}\text{.of lps}$Bps refers to bond pairs and lps refers to lone pairs and the steric number obtained will give an idea about the hybridization of the molecule.Recently Updated PagesMaster Class 11 Business Studies: Engaging Questions & Answers for Success
$N{{H}_{3}}$ The hybridization of this adduct formed is $s{{p}^{3}}$ hybridized adduct.Note: The hybridization of a molecule is calculate using the formulae,$\text{steric number = No}\text{. of bps + No}\text{.of lps}$Bps refers to bond pairs and lps refers to lone pairs and the steric number obtained will give an idea about the hybridization of the molecule.Recently Updated PagesMaster Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Chemistry: Engaging Questions & Answers for Success
Master Class 11 Chemistry: Engaging Questions & Answers for Success Master Class 11 Business Studies: Engaging Questions & Answers for Success
Master Class 11 Business Studies: Engaging Questions & Answers for Success Master Class 11 Computer Science: Engaging Questions & Answers for Success
Master Class 11 Computer Science: Engaging Questions & Answers for Success Master Class 11 Economics: Engaging Questions & Answers for Success
Master Class 11 Economics: Engaging Questions & Answers for Success Master Class 11 Social Science: Engaging Questions & Answers for Success
Master Class 11 Social Science: Engaging Questions & Answers for Success Master Class 11 English: Engaging Questions & Answers for Success
Master Class 11 English: Engaging Questions & Answers for Success Master Class 11 Chemistry: Engaging Questions & Answers for Success
Master Class 11 Chemistry: Engaging Questions & Answers for Success
- 1
- 2
 The period of a conical pendulum in terms of its length class 11 physics CBSE
The period of a conical pendulum in terms of its length class 11 physics CBSE One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE
In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE What is the difference between free and forced vib class 11 physics CBSE
What is the difference between free and forced vib class 11 physics CBSE A large number of liquid drops each of radius r coalesce class 11 physics CBSE
A large number of liquid drops each of radius r coalesce class 11 physics CBSE The period of a conical pendulum in terms of its length class 11 physics CBSE
The period of a conical pendulum in terms of its length class 11 physics CBSE One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE
In a fight of 600km an aircraft was slowed down du-class-11-maths-CBSE State and prove Bernoullis theorem class 11 physics CBSE
State and prove Bernoullis theorem class 11 physics CBSE What is the difference between free and forced vib class 11 physics CBSE
What is the difference between free and forced vib class 11 physics CBSE
- 1
- 2
Repeaters Course for NEET 2022 - 23
NEET Repeater 2023 - Aakrosh 1 Year CourseTag » When Bf3 And Nh3 Are Mixed
-
Why Do NH3 And BF3 Form An Adduct Readily? - Quora
-
The Formation Of Molecular Complex BF3 - Hybridization - Toppr
-
Boron Trifluoride, BF3, Reacts With Ammonia, NH3, To Form An Addition ...
-
The Formation Of Molecular Complex BF3 NH3 Results Class 11 ...
-
Hybridization Of NH3 And BF3 Adduct - YouTube
-
What Happens When BF3 Is Treated With NH3 Answer Soon
-
[PDF] Molecular Orbital And Density Functional Study Of The Formation ...
-
Boron Trifluoride - Wikipedia
-
Is There Any Change In The Hybridisation Of B And N Atoms As A Result ...
-
[DOC] Final Exam Review - Dean Of Students Office | Iowa State University
-
Boron Trifluoride BF3 Is A Nonpolar Molecule Whereas Ammonia ...
-
Boron Trifluoride | Chemical Compound - Britannica
-
An Ab Initio Study Of The Complex BF3·NF3 - ACS Publications