21.4: Acidity And Basicity Of Amines - Chemistry LibreTexts
Acidity of Amines
We normally think of amines as bases, but it must be remembered that 1º and 2º-amines are also very weak acids (ammonia has a pKa = 34). In this respect it should be noted that pKa is being used as a measure of the acidity of the amine itself rather than its conjugate acid, as in the previous section. For ammonia this is expressed by the following hypothetical equation:
NH3 + H2O ____> NH2(–) + H2O-H(+)
The same factors that decreased the basicity of amines increase their acidity. This is illustrated by the following examples, which are shown in order of increasing acidity. It should be noted that the first four examples have the same order and degree of increased acidity as they exhibited decreased basicity in the previous table. The first compound is a typical 2º-amine, and the three next to it are characterized by varying degrees of nitrogen electron pair delocalization. The last two compounds (shaded blue) show the influence of adjacent sulfonyl and carbonyl groups on N-H acidity. From previous discussion it should be clear that the basicity of these nitrogens is correspondingly reduced.
| Compound | 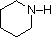 | 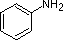 |  | 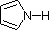 | C6H5SO2NH2 | 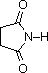 |
|---|---|---|---|---|---|---|
| pKa | 33 | 27 | 19 | 15 | 10 | 9.6 |
The acids shown here may be converted to their conjugate bases by reaction with bases derived from weaker acids (stronger bases). Three examples of such reactions are shown below, with the acidic hydrogen colored red in each case. For complete conversion to the conjugate base, as shown, a reagent base roughly a million times stronger is required.
C6H5SO2NH2 + KOH  C6H5SO2NH(–) K(+) + H2O C6H5SO2NH(–) K(+) + H2O | a sulfonamide base |
(CH3)3COH + NaH  (CH3)3CO(–) Na(+) + H2 (CH3)3CO(–) Na(+) + H2 | an alkoxide base |
(C2H5)2NH + C4H9Li  (C2H5)2N(–) Li(+) + C4H10 (C2H5)2N(–) Li(+) + C4H10 | an amide base |
Từ khóa » Nh2 Vs Nh3 Pka
-
[PDF] Amine Basicity Of Amine NH2 < N H N < Ammonium PKa NH2
-
[PDF] Amines. Organic Derivatives Of Ammonia, NH3. Nitrogen Atoms Have ...
-
[PDF] Approximate PKa Values (rounded To Nearest 5)
-
Is NH2 More Acidic Than NH3?
-
[PDF] PKa Table.1
-
How To Use A PKa Table - Master Organic Chemistry
-
Amine Basicity Is Measued By The PKa Of Its Conjugate Acid (pKaH)
-
[PDF] 23.5 BASICITY AND ACIDITY OF AMINES
-
[PDF] CHEM1611 Answers To Problem Sheet 10
-
Which Has More Acidic Strength, NH3 Or OH? - Quora
-
[PDF] Chem 109 C
-
[PDF] PKa Chart - Utah Tech University
-
[PDF] Chapter 6 Amines And Amides - Angelo State University