| Author: | Subject: Ammonia from sodium hydroxide & urea. what would be the by-product cyanide or carbonate? |
bluamine Hazard to Others    Posts: 197 Registered: 17-8-2015 Member Is Offline Mood: No Mood Posts: 197 Registered: 17-8-2015 Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 29-3-2016 at 07:38 posted on 29-3-2016 at 07:38 | |
Ammonia from sodium hydroxide & urea. what would be the by-product cyanide or carbonate? Hi everyone!! I read before some threads on the forum here claiming this reaction would give sodium carbonate, but recently i watched a Nurdrage's video about making sodium cyanide claiming there's an alternative method (which he didn't use) to make it using both of those reactives (NaOH & CH4ON2)!! It's only because i really respect that guy & believe he's not an amateur or beginner, that i am still comfused about this subject. I suppose sodium cyanide is may be dangerous so i hope i will be able to avoid it. Need your help please  |
| |
macckone Dispenser of practical lab wisdom      Posts: 2211 Registered: 1-3-2013 Location: Over a mile high Member Is Offline Mood: Electrical Posts: 2211 Registered: 1-3-2013 Location: Over a mile high Member Is Offline Mood: Electrical | ![[*]](images/xpblue/default_icon.gif) posted on 29-3-2016 at 09:34 posted on 29-3-2016 at 09:34 | |
| http://www.sciencemadness.org/talk/viewthread.php?tid=19575 given sufficient hydroxide it should proceed to carbonate. with otherwise it stops at sodium cyanide. this is not a 100% thing as most chemical reactions there are equilibrium involved including those with water. small amounts of cyanide will be released in any case. |
| |
unionised International Hazard      Posts: 5142 Registered: 1-11-2003 Location: UK Member Is Offline Mood: No Mood Posts: 5142 Registered: 1-11-2003 Location: UK Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 29-3-2016 at 10:31 posted on 29-3-2016 at 10:31 | |
| Is cyanide a plausible by-product in aqueous solution?# |
| |
bluamine Hazard to Others    Posts: 197 Registered: 17-8-2015 Member Is Offline Mood: No Mood Posts: 197 Registered: 17-8-2015 Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 29-3-2016 at 11:26 posted on 29-3-2016 at 11:26 | |
Quote: Originally posted by unionised  | | Is cyanide a plausible by-product in aqueous solution?# |
I think the process should be done by dry distillation |
| |
macckone Dispenser of practical lab wisdom      Posts: 2211 Registered: 1-3-2013 Location: Over a mile high Member Is Offline Mood: Electrical Posts: 2211 Registered: 1-3-2013 Location: Over a mile high Member Is Offline Mood: Electrical | ![[*]](images/xpblue/default_icon.gif) posted on 29-3-2016 at 19:03 posted on 29-3-2016 at 19:03 | |
| The initial step yields water, sodium cyanide and ammonia. Sodium cyanide and water will exist in equilibrium with hydrogen cyanide and sodium hydroxide. Sodium cyanide, water, and sodium hydroxide will form sodium carbonate and ammonia but this is the slow step. A large excess of sodium hydroxide hydrate reduces hydrogen cyanide formation. |
| |
Fluorite Hazard to Others    Posts: 142 Registered: 26-12-2018 Member Is Offline Posts: 142 Registered: 26-12-2018 Member Is Offline | ![[*]](images/xpblue/default_icon.gif) posted on 10-11-2020 at 12:06 posted on 10-11-2020 at 12:06 | |
| Yo wtf you can't make sodium cyanide by just mixing urea and NaOH! But probably you can get some cyanates and they usually decompose to ammonium carbonate |
| |
Swinfi2 Hazard to Others    Posts: 132 Registered: 19-2-2018 Location: England Member Is Offline Mood: Catalytic Posts: 132 Registered: 19-2-2018 Location: England Member Is Offline Mood: Catalytic | ![[*]](images/xpblue/default_icon.gif) posted on 10-11-2020 at 15:42 posted on 10-11-2020 at 15:42 | |
| I'm going to say it isn't so plausible imo to get cyanide from this. People have been making ammonia from urea and NaOH for at least a while and as far as I know none of them seem to be worried about leaving a still pot full of sodium cyanide. Also people have been making cyanide in different ways for at least a while and if this was a legit process it would be a super easy well known route. (Right?) |
| |
ArbuzToWoda Hazard to Self   Posts: 98 Registered: 15-7-2020 Member Is Offline Posts: 98 Registered: 15-7-2020 Member Is Offline | ![[*]](images/xpblue/default_icon.gif) posted on 10-11-2020 at 15:45 posted on 10-11-2020 at 15:45 | |
Obviously no cyanides are produced in this reaction. It is a very messy one, yes, leading to formation of many organic and inorganic byproducts, but it is mostly CO2 and ammonia. 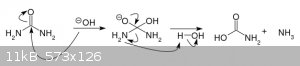 [Edited on 10-11-2020 by ArbuzToWoda] [Edited on 10-11-2020 by ArbuzToWoda] |
| |
karlos³ International Hazard      Posts: 1520 Registered: 10-1-2011 Location: yes! Member Is Offline Mood: oxazolidinic 8) Posts: 1520 Registered: 10-1-2011 Location: yes! Member Is Offline Mood: oxazolidinic 8) | ![[*]](images/xpblue/default_icon.gif) posted on 10-11-2020 at 16:11 posted on 10-11-2020 at 16:11 | |
| Biuret! But otherwise, properly purified, the cyanate is good enough to make cyanide from. |
| |
macckone Dispenser of practical lab wisdom      Posts: 2211 Registered: 1-3-2013 Location: Over a mile high Member Is Offline Mood: Electrical Posts: 2211 Registered: 1-3-2013 Location: Over a mile high Member Is Offline Mood: Electrical | ![[*]](images/xpblue/default_icon.gif) posted on 10-11-2020 at 21:58 posted on 10-11-2020 at 21:58 | |
| If the reaction is performed dry you get cyanate that rearranges to cyanide as the temperature rises. If the reaction is performed in water you get cyanate which produces some cyanide but mostly decomposes to carbonate and ammonia. Everything is an equilibrium, water shifts the equilibrium as does sodium hydroxide. My initial post was incorrect, the intermediate is cyanate not cyanide. However under dry conditions cyanide is formed. There is another post on that specific procedure and it requires high temperature to get substantially complete conversion. |
| |

 Search
Search  FAQ
FAQ  Member List
Member List  Today's Posts
Today's Posts  Stats
Stats 






