Ammonia, NH3 And Hydrazine, NH2NH2, Are Two Compounds Of ...
CoursesCourses for KidsFree study materialOffline CentresMore Store
Store

 Answer
Answer Question Answers for Class 12
Question Answers for Class 12 Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11
Class 12 BiologyClass 12 ChemistryClass 12 EnglishClass 12 MathsClass 12 PhysicsClass 12 Social ScienceClass 12 Business StudiesClass 12 EconomicsQuestion Answers for Class 11 Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10
Class 11 EconomicsClass 11 Computer ScienceClass 11 BiologyClass 11 ChemistryClass 11 EnglishClass 11 MathsClass 11 PhysicsClass 11 Social ScienceClass 11 AccountancyClass 11 Business StudiesQuestion Answers for Class 10 Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9
Class 10 ScienceClass 10 EnglishClass 10 MathsClass 10 Social ScienceClass 10 General KnowledgeQuestion Answers for Class 9 Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8
Class 9 General KnowledgeClass 9 ScienceClass 9 EnglishClass 9 MathsClass 9 Social ScienceQuestion Answers for Class 8 Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7
Class 8 ScienceClass 8 EnglishClass 8 MathsClass 8 Social ScienceQuestion Answers for Class 7 Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6
Class 7 ScienceClass 7 EnglishClass 7 MathsClass 7 Social ScienceQuestion Answers for Class 6 Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5
Class 6 ScienceClass 6 EnglishClass 6 MathsClass 6 Social ScienceQuestion Answers for Class 5 Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4
Class 5 ScienceClass 5 EnglishClass 5 MathsClass 5 Social ScienceQuestion Answers for Class 4 Class 4 ScienceClass 4 EnglishClass 4 Maths
Class 4 ScienceClass 4 EnglishClass 4 Maths
 Ammonia, $N{H_3}$ and hydrazine, $N{H_2}N{H_2}$, are two compounds of nitrogen, ${N_2}$. Which statement is correct?A.The N-N bond in $N{H_2} - N{H_2}$ is polar.B.$N{H_3}$ and $N{H_2}N{H_2}$ have lone pairs of electrons but ${N_2}$ does not. C.The oxidation number of each nitrogen in $N{H_2}N{H_2}$ is +2.D. The reaction of nitrogen with hydrogen has a high activation rate.Answer
Ammonia, $N{H_3}$ and hydrazine, $N{H_2}N{H_2}$, are two compounds of nitrogen, ${N_2}$. Which statement is correct?A.The N-N bond in $N{H_2} - N{H_2}$ is polar.B.$N{H_3}$ and $N{H_2}N{H_2}$ have lone pairs of electrons but ${N_2}$ does not. C.The oxidation number of each nitrogen in $N{H_2}N{H_2}$ is +2.D. The reaction of nitrogen with hydrogen has a high activation rate.Answer Verified595.5k+ viewsHint: The N-H bond is strong due to the high electronegativity of N atoms and so a large amount of energy is required to break the bond (approximately 386 kJ/mol). Now try all the options and see which one is correct.Complete step by step answer:-In$N{H_2}N{H_2}$, since both the nitrogen atoms have the same value of electronegativity the bond between the 2 nitrogen atoms (N-N) will be non-polar. This contradicts option (A), so option (A) is false.-In $N{H_3}$, out of the 5 electrons from the outermost shell of N, 3 have bonded with 1 Hydrogen atom each. Hence leaving behind a lone pair of electrons.In $N{H_2} - N{H_2}$, for each nitrogen atom having 5 outer shell electrons, 2 are bonded with 1 hydrogen each and with one electron they bond with an electron from the other nitrogen. Hence each of the nitrogen is left with a lone pair of electrons.In${N_2}$, the 2 N atoms are bonded to each other by a triple bond and so leaving behind a lone pair of electrons each.
Verified595.5k+ viewsHint: The N-H bond is strong due to the high electronegativity of N atoms and so a large amount of energy is required to break the bond (approximately 386 kJ/mol). Now try all the options and see which one is correct.Complete step by step answer:-In$N{H_2}N{H_2}$, since both the nitrogen atoms have the same value of electronegativity the bond between the 2 nitrogen atoms (N-N) will be non-polar. This contradicts option (A), so option (A) is false.-In $N{H_3}$, out of the 5 electrons from the outermost shell of N, 3 have bonded with 1 Hydrogen atom each. Hence leaving behind a lone pair of electrons.In $N{H_2} - N{H_2}$, for each nitrogen atom having 5 outer shell electrons, 2 are bonded with 1 hydrogen each and with one electron they bond with an electron from the other nitrogen. Hence each of the nitrogen is left with a lone pair of electrons.In${N_2}$, the 2 N atoms are bonded to each other by a triple bond and so leaving behind a lone pair of electrons each. 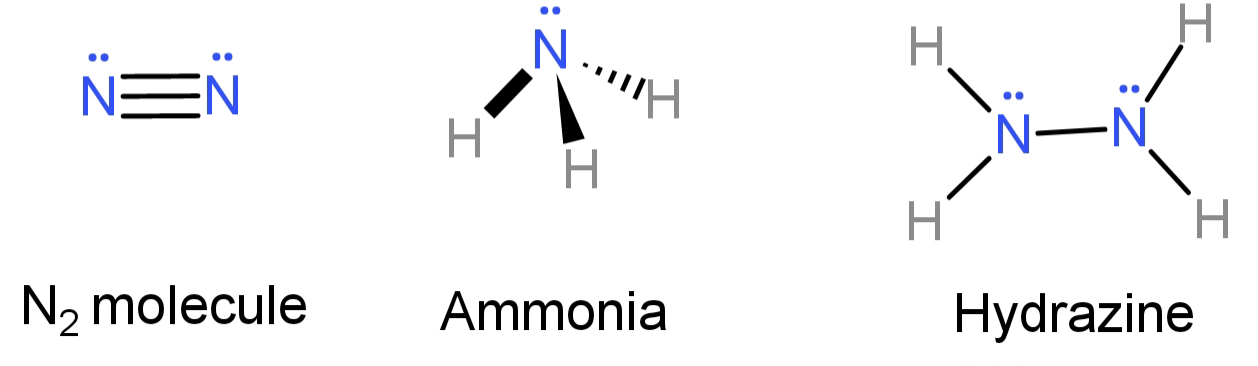 Hence we now know that all three of them have lone pairs of electrons. This conclusion contradicts option (B), so we can say that option (B) is false.-In $N{H_2}N{H_2}$: to calculate the oxidation state (O.s.) of N atoms let us assume it to be ‘x’ and the oxidation state of H is already equal to 1. So: O.s. of 2 N + O.s. of 4 H = O.s. of entire molecule (Total oxidation state of the molecule is 0 because the molecule is electrically neutral 2x + 4 = 0 and so x = -2Hence the oxidation state of each nitrogen here is (-2). This contradicts option (C), so option (C) is also false.-The reaction of nitrogen with hydrogen has a high activation rate due to the high bond energy between them (which was due to high electronegativity of N).Hence, the correct option is: (D).Note:Nitrogen reacts with hydrogen only under high temperature and pressure, that too in the presence of a metal catalyst to overcome the high activation energy. It is a type of combination reaction and produces ammonia. This process is also known as Haber’s process.Recently Updated PagesThe total number of structural isomers possible for class 12 chemistry CBSE
Hence we now know that all three of them have lone pairs of electrons. This conclusion contradicts option (B), so we can say that option (B) is false.-In $N{H_2}N{H_2}$: to calculate the oxidation state (O.s.) of N atoms let us assume it to be ‘x’ and the oxidation state of H is already equal to 1. So: O.s. of 2 N + O.s. of 4 H = O.s. of entire molecule (Total oxidation state of the molecule is 0 because the molecule is electrically neutral 2x + 4 = 0 and so x = -2Hence the oxidation state of each nitrogen here is (-2). This contradicts option (C), so option (C) is also false.-The reaction of nitrogen with hydrogen has a high activation rate due to the high bond energy between them (which was due to high electronegativity of N).Hence, the correct option is: (D).Note:Nitrogen reacts with hydrogen only under high temperature and pressure, that too in the presence of a metal catalyst to overcome the high activation energy. It is a type of combination reaction and produces ammonia. This process is also known as Haber’s process.Recently Updated PagesThe total number of structural isomers possible for class 12 chemistry CBSE What is a parallel plate capacitor Deduce the expression class 12 physics CBSE
What is a parallel plate capacitor Deduce the expression class 12 physics CBSE Master Class 12 Social Science: Engaging Questions & Answers for Success
Master Class 12 Social Science: Engaging Questions & Answers for Success Master Class 12 Physics: Engaging Questions & Answers for Success
Master Class 12 Physics: Engaging Questions & Answers for Success Master Class 12 Maths: Engaging Questions & Answers for Success
Master Class 12 Maths: Engaging Questions & Answers for Success Master Class 12 Economics: Engaging Questions & Answers for Success
Master Class 12 Economics: Engaging Questions & Answers for Success The total number of structural isomers possible for class 12 chemistry CBSE
The total number of structural isomers possible for class 12 chemistry CBSE What is a parallel plate capacitor Deduce the expression class 12 physics CBSE
What is a parallel plate capacitor Deduce the expression class 12 physics CBSE Master Class 12 Social Science: Engaging Questions & Answers for Success
Master Class 12 Social Science: Engaging Questions & Answers for Success Master Class 12 Physics: Engaging Questions & Answers for Success
Master Class 12 Physics: Engaging Questions & Answers for Success Master Class 12 Maths: Engaging Questions & Answers for Success
Master Class 12 Maths: Engaging Questions & Answers for Success Master Class 12 Economics: Engaging Questions & Answers for Success
Master Class 12 Economics: Engaging Questions & Answers for Success
 What are the major means of transport Explain each class 12 social science CBSE
What are the major means of transport Explain each class 12 social science CBSE Draw a labelled sketch of the human eye class 12 physics CBSE
Draw a labelled sketch of the human eye class 12 physics CBSE What is a transformer Explain the principle construction class 12 physics CBSE
What is a transformer Explain the principle construction class 12 physics CBSE Differentiate between insitu conservation and exsitu class 12 biology CBSE
Differentiate between insitu conservation and exsitu class 12 biology CBSE Who is Mukesh What is his dream Why does it look like class 12 english CBSE
Who is Mukesh What is his dream Why does it look like class 12 english CBSE Which are the Top 10 Largest Countries of the World?
Which are the Top 10 Largest Countries of the World? What are the major means of transport Explain each class 12 social science CBSE
What are the major means of transport Explain each class 12 social science CBSE Draw a labelled sketch of the human eye class 12 physics CBSE
Draw a labelled sketch of the human eye class 12 physics CBSE What is a transformer Explain the principle construction class 12 physics CBSE
What is a transformer Explain the principle construction class 12 physics CBSE Differentiate between insitu conservation and exsitu class 12 biology CBSE
Differentiate between insitu conservation and exsitu class 12 biology CBSE Who is Mukesh What is his dream Why does it look like class 12 english CBSE
Who is Mukesh What is his dream Why does it look like class 12 english CBSE
Talk to our experts
1800-120-456-456
Sign In- Question Answer
- Class 12
- Chemistry
- Ammonia NH3 and hydrazine NH2N...


 Question Answers for Class 12
Question Answers for Class 12
 Ammonia, $N{H_3}$ and hydrazine, $N{H_2}N{H_2}$, are two compounds of nitrogen, ${N_2}$. Which statement is correct?A.The N-N bond in $N{H_2} - N{H_2}$ is polar.B.$N{H_3}$ and $N{H_2}N{H_2}$ have lone pairs of electrons but ${N_2}$ does not. C.The oxidation number of each nitrogen in $N{H_2}N{H_2}$ is +2.D. The reaction of nitrogen with hydrogen has a high activation rate.Answer
Ammonia, $N{H_3}$ and hydrazine, $N{H_2}N{H_2}$, are two compounds of nitrogen, ${N_2}$. Which statement is correct?A.The N-N bond in $N{H_2} - N{H_2}$ is polar.B.$N{H_3}$ and $N{H_2}N{H_2}$ have lone pairs of electrons but ${N_2}$ does not. C.The oxidation number of each nitrogen in $N{H_2}N{H_2}$ is +2.D. The reaction of nitrogen with hydrogen has a high activation rate.Answer Hence we now know that all three of them have lone pairs of electrons. This conclusion contradicts option (B), so we can say that option (B) is false.-In $N{H_2}N{H_2}$: to calculate the oxidation state (O.s.) of N atoms let us assume it to be ‘x’ and the oxidation state of H is already equal to 1. So: O.s. of 2 N + O.s. of 4 H = O.s. of entire molecule (Total oxidation state of the molecule is 0 because the molecule is electrically neutral 2x + 4 = 0 and so x = -2Hence the oxidation state of each nitrogen here is (-2). This contradicts option (C), so option (C) is also false.-The reaction of nitrogen with hydrogen has a high activation rate due to the high bond energy between them (which was due to high electronegativity of N).Hence, the correct option is: (D).Note:Nitrogen reacts with hydrogen only under high temperature and pressure, that too in the presence of a metal catalyst to overcome the high activation energy. It is a type of combination reaction and produces ammonia. This process is also known as Haber’s process.Recently Updated PagesThe total number of structural isomers possible for class 12 chemistry CBSE
Hence we now know that all three of them have lone pairs of electrons. This conclusion contradicts option (B), so we can say that option (B) is false.-In $N{H_2}N{H_2}$: to calculate the oxidation state (O.s.) of N atoms let us assume it to be ‘x’ and the oxidation state of H is already equal to 1. So: O.s. of 2 N + O.s. of 4 H = O.s. of entire molecule (Total oxidation state of the molecule is 0 because the molecule is electrically neutral 2x + 4 = 0 and so x = -2Hence the oxidation state of each nitrogen here is (-2). This contradicts option (C), so option (C) is also false.-The reaction of nitrogen with hydrogen has a high activation rate due to the high bond energy between them (which was due to high electronegativity of N).Hence, the correct option is: (D).Note:Nitrogen reacts with hydrogen only under high temperature and pressure, that too in the presence of a metal catalyst to overcome the high activation energy. It is a type of combination reaction and produces ammonia. This process is also known as Haber’s process.Recently Updated PagesThe total number of structural isomers possible for class 12 chemistry CBSE What is a parallel plate capacitor Deduce the expression class 12 physics CBSE
What is a parallel plate capacitor Deduce the expression class 12 physics CBSE Master Class 12 Social Science: Engaging Questions & Answers for Success
Master Class 12 Social Science: Engaging Questions & Answers for Success Master Class 12 Physics: Engaging Questions & Answers for Success
Master Class 12 Physics: Engaging Questions & Answers for Success Master Class 12 Maths: Engaging Questions & Answers for Success
Master Class 12 Maths: Engaging Questions & Answers for Success Master Class 12 Economics: Engaging Questions & Answers for Success
Master Class 12 Economics: Engaging Questions & Answers for Success The total number of structural isomers possible for class 12 chemistry CBSE
The total number of structural isomers possible for class 12 chemistry CBSE What is a parallel plate capacitor Deduce the expression class 12 physics CBSE
What is a parallel plate capacitor Deduce the expression class 12 physics CBSE Master Class 12 Social Science: Engaging Questions & Answers for Success
Master Class 12 Social Science: Engaging Questions & Answers for Success Master Class 12 Physics: Engaging Questions & Answers for Success
Master Class 12 Physics: Engaging Questions & Answers for Success Master Class 12 Maths: Engaging Questions & Answers for Success
Master Class 12 Maths: Engaging Questions & Answers for Success Master Class 12 Economics: Engaging Questions & Answers for Success
Master Class 12 Economics: Engaging Questions & Answers for Success
- 1
- 2
 What are the major means of transport Explain each class 12 social science CBSE
What are the major means of transport Explain each class 12 social science CBSE Draw a labelled sketch of the human eye class 12 physics CBSE
Draw a labelled sketch of the human eye class 12 physics CBSE What is a transformer Explain the principle construction class 12 physics CBSE
What is a transformer Explain the principle construction class 12 physics CBSE Differentiate between insitu conservation and exsitu class 12 biology CBSE
Differentiate between insitu conservation and exsitu class 12 biology CBSE Who is Mukesh What is his dream Why does it look like class 12 english CBSE
Who is Mukesh What is his dream Why does it look like class 12 english CBSE Which are the Top 10 Largest Countries of the World?
Which are the Top 10 Largest Countries of the World? What are the major means of transport Explain each class 12 social science CBSE
What are the major means of transport Explain each class 12 social science CBSE Draw a labelled sketch of the human eye class 12 physics CBSE
Draw a labelled sketch of the human eye class 12 physics CBSE What is a transformer Explain the principle construction class 12 physics CBSE
What is a transformer Explain the principle construction class 12 physics CBSE Differentiate between insitu conservation and exsitu class 12 biology CBSE
Differentiate between insitu conservation and exsitu class 12 biology CBSE Who is Mukesh What is his dream Why does it look like class 12 english CBSE
Who is Mukesh What is his dream Why does it look like class 12 english CBSE
- 1
- 2
Repeaters Course for NEET 2022 - 23
NEET Repeater 2023 - Aakrosh 1 Year CourseTừ khóa » Nh2.nh2 Oxidation Number
-
NH2NH2 Oxidation Number - ChemicalAid
-
Ammonia, NH3 - Are Two Compounds Of Nitrogen, N2 - Toppr
-
How To Find The Oxidation Number For N In The NH2 - Ion. - YouTube
-
What Is The Oxidation State Of Nitrogen In Hydrozine? - Quora
-
What Is The Oxidation Number Of Nitrogen In The NH2NH2 Molecule?
-
In Which Pair Of Compounds The Oxidation State Of Nitrogen Is - Byjus
-
The Oxidation State Of Nitrogen In Hydrazine Is - Doubtnut
-
What Is The Oxidation Number Of Nitrogen In The NH2NH2 Molecule?
-
Oxidation State Of Each Nitrogen In Nh2-nh2 Is 1)-2
-
Oxidation State Of Each Nitrogen In NH2-NH2 Is 1)-2 - Meritnation
-
Understanding Hydrazine Oxidation Electrocatalysis On Undoped ...
-
What Is The Oxidation State Of Nitrogen In Hydrazine, N2H4?
-
Hydrazine - Wikipedia