And C24:0-dihydroceramides Confer Mixed Cytotoxicity In T-cell ...
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation Search: Search Advanced Clipboard Save Email Send to
Search: Search Advanced Clipboard Save Email Send to- Clipboard
- My Bibliography
- Collections
- Citation manager
Save citation to file
Format: Summary (text) PubMed PMID Abstract (text) CSV Create file CancelEmail citation
Email address has not been verified. Go to My NCBI account settings to confirm your email and then refresh this page. To: Subject: Body: Format: Summary Summary (text) Abstract Abstract (text) MeSH and other data Send email CancelAdd to Collections
- Create a new collection
- Add to an existing collection
Add to My Bibliography
- My Bibliography
Your saved search
Name of saved search: Search terms: Test search terms Would you like email updates of new search results? Saved Search Alert Radio Buttons- Yes
- No
Create a file for external citation management software
Create file CancelYour RSS Feed
Name of RSS Feed: Number of items displayed: 5 10 15 20 50 100 Create RSS Cancel RSS Link CopyFull text links
 Public Library of Science Free PMC article Full text links
Public Library of Science Free PMC article Full text links Actions
CiteCollectionsAdd to Collections- Create a new collection
- Add to an existing collection
Page navigation
- Title & authors
- Abstract
- Conflict of interest statement
- Figures
- References
- Publication types
- MeSH terms
- Substances
- Grants and funding
- LinkOut - more resources
Abstract
We previously reported that fenretinide (4-HPR) was cytotoxic to acute lymphoblastic leukemia (ALL) cell lines in vitro in association with increased levels of de novo synthesized dihydroceramides, the immediate precursors of ceramides. However, the cytotoxic potentials of native dihydroceramides have not been defined. Therefore, we determined the cytotoxic effects of increasing dihydroceramide levels via de novo synthesis in T-cell ALL cell lines and whether such cytotoxicity was dependent on an absolute increase in total dihydroceramide mass versus an increase of certain specific dihydroceramides. A novel method employing supplementation of individual fatty acids, sphinganine, and the dihydroceramide desaturase-1 (DES) inhibitor, GT-11, was used to increase de novo dihydroceramide synthesis and absolute levels of specific dihydroceramides and ceramides. Sphingolipidomic analyses of four T-cell ALL cell lines revealed strong positive correlations between cytotoxicity and levels of C22:0-dihydroceramide (ρ = 0.74-0.81, P ≤ 0.04) and C24:0-dihydroceramide (ρ = 0.84-0.90, P ≤ 0.004), but not between total or other individual dihydroceramides, ceramides, or sphingoid bases or phosphorylated derivatives. Selective increase of C22:0- and C24:0-dihydroceramide increased level and flux of autophagy marker, LC3B-II, and increased DNA fragmentation (TUNEL assay) in the absence of an increase of reactive oxygen species; pan-caspase inhibition blocked DNA fragmentation but not cell death. C22:0-fatty acid supplemented to 4-HPR treated cells further increased C22:0-dihydroceramide levels (P ≤ 0.001) and cytotoxicity (P ≤ 0.001). These data demonstrate that increases of specific dihydroceramides are cytotoxic to T-cell ALL cells by a caspase-independent, mixed cell death mechanism associated with increased autophagy and suggest that dihydroceramides may contribute to 4-HPR-induced cytotoxicity. The targeted increase of specific acyl chain dihydroceramides may constitute a novel anticancer approach.
PubMed Disclaimer
Conflict of interest statement
Competing Interests: SBC is employed by Research and Testing Laboratory, LLC. A patent application by the Texas Tech University System (TTUS) is pending, on which BJM is an Inventor. As TTUS has a revenue-sharing policy for faculty-inventors, should this patent be issued and commercialized, BJM may share in patent-derived revenues. Patent application: Num. 20120121691 A1, dated May 17, 2012, entitled, " Method for Increasing the Production of a Specific ACYL-Chain Dihydroceramide(s) for Improving the Effectiveness of Cancer Treatments." Assignee: Texas Tech University System. There are no further patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures
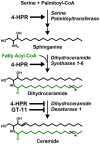
Figure 1. Schematic of the de novo …
Figure 1. Schematic of the de novo ceramide pathway.
Rate-limiting enzyme, serine palmitoyltransferase (SPT), condenses…

Figure 2. Effects of sphinganine and GT-11…
Figure 2. Effects of sphinganine and GT-11 on dihydroceramides, ceramides and cytotoxicity.
A - C …
Figure 3. Effects of specific fatty acids…
Figure 3. Effects of specific fatty acids on sphinganine ± GT-11-induced dihydroceramides and cytotoxicity.
A …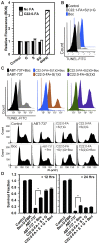
Figure 4. Mechanisms of C22:0-dihydroceramide induced cell…
Figure 4. Mechanisms of C22:0-dihydroceramide induced cell death.
A ) Reactive oxygen species levels in…
Figure 5. Caspase cleavage and LC3B-I/II turnover.
Figure 5. Caspase cleavage and LC3B-I/II turnover.
A ) CCRF-CEM cells treated with drug/fatty acid…
Figure 6. Effects of specific fatty acids…
Figure 6. Effects of specific fatty acids on sphinganine + GT-11-induced dihydroceramide accumulation and cytotoxicity.

Figure 7. Effects of specific fatty acids…
Figure 7. Effects of specific fatty acids on 4-HPR-induced dihydroceramide levels and cytotoxicity.
A )…References
-
- Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F et al. (1993) N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res 53: 6036-6041. PubMed: 8261419. - PubMed
-
- O’Donnell PH, Guo WX, Reynolds CP, Maurer BJ (2002) N-(4-hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia 16: 902-910. doi:10.1038/sj.leu.2402485. PubMed: 11986953. - DOI - PubMed
-
- Faderl S, Lotan R, Kantarjian HM, Harris D, Van Q et al. (2003) N-(4-Hydroxylphenyl)retinamide (fenretinide, 4-HPR), a retinoid compound with antileukemic and proapoptotic activity in acute lymphoblastic leukemia (ALL). Leuk Res 27: 259-266. doi:10.1016/S0145-2126(02)00162-5. PubMed: 12537979. - DOI - PubMed
-
- Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP (2008) Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst 100: 580-595. doi:10.1093/jnci/djn076. PubMed: 18398104. - DOI - PubMed
-
- Oridate N, Suzuki S, Higuchi M, Mitchell MF, Hong WK et al. (1997) Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J Natl Cancer Inst 89: 1191-1198. doi:10.1093/jnci/89.16.1191. PubMed: 9274913. - DOI - PubMed
Publication types
- Research Support, N.I.H., Extramural Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Research Support, Non-U.S. Gov't Actions
- Search in PubMed
- Search in MeSH
- Add to Search
MeSH terms
- Antineoplastic Agents / chemistry Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Autophagy Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Cell Line, Tumor Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Ceramides / chemistry* Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Culture Media / chemistry Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- DNA Fragmentation Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Fatty Acids / chemistry Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Humans Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Mitochondria / metabolism Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Precursor T-Cell Lymphoblastic Leukemia-Lymphoma / drug therapy* Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Precursor T-Cell Lymphoblastic Leukemia-Lymphoma / metabolism* Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Reactive Oxygen Species Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Sphingolipids / chemistry Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Sphingosine / analogs & derivatives Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Sphingosine / chemistry Actions
- Search in PubMed
- Search in MeSH
- Add to Search
Substances
- Antineoplastic Agents Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Ceramides Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Culture Media Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Fatty Acids Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Reactive Oxygen Species Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Sphingolipids Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- dihydroceramide Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Sphingosine Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- safingol Actions
- Search in PubMed
- Search in MeSH
- Add to Search
Grants and funding
- R01 CA100895/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
- Europe PubMed Central
- PubMed Central
- Public Library of Science
Other Literature Sources
- scite Smart Citations
Miscellaneous
- NCI CPTAC Assay Portal
 Public Library of Science Free PMC article [x] Cite Copy Download .nbib .nbib Format: AMA APA MLA NLM Send To
Public Library of Science Free PMC article [x] Cite Copy Download .nbib .nbib Format: AMA APA MLA NLM Send To - Clipboard
- Save
- My Bibliography
- Collections
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Từ khóa » C24.0
-
C24.0 - Malignant Neoplasm Of Extrahepatic Bile Duct | ICD-10-CM
-
2022 ICD-10-CM Diagnosis Code C24.0: Malignant Neoplasm Of ...
-
C24:0 And C24:1 Sphingolipids In Cholesterol-containing, Five - Nature
-
C24.9 - ICD-10 Version:2019
-
C24:0 Avoids Cold Exposure-induced Oxidative Stress And Fatty Acid ...
-
C24.0 Bösartige Neubildung: Extrahepatischer Gallengang
-
Lignoceric Acid - Wikipedia
-
2-Hydroxytetracosanoic Acid, 2-Hydroxy C24:0 Fatty Acid (ab144018)
-
ICD-10 Code For Malignant Neoplasm Of Extrahepatic Bile Duct- C24.0
-
Lignoceric Acid Methyl Ester - Cayman Chemical
-
Lignoceric Acid-d 47 - Cayman Chemical
-
Fatty ry Long Chain C24:0 (Tetracosanoate)/C22:0 ... - LOINC
-
Fatty ry Long Chain C24:0 (Tetracosanoate) [Moles ... - LOINC