And Ca2UO2(CO3)3(aq) Complexes At Variable Temperatures (10 ...
- Previous Article
- Next Article
 From the journal:
From the journal: Dalton Transactions
Formation of CaUO2(CO3)32− and Ca2UO2(CO3)3(aq) complexes at variable temperatures (10–70 °C)†
 c and Jong-Il Yun
c and Jong-Il Yun  *a Author affiliations
*a Author affiliations * Corresponding authors
a Department of Nuclear and Quantum Engineering, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon 34141, Republic of Korea E-mail: [email protected]
b Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Japan
c Nuclear Chemistry Research Division, Korea Atomic Energy Research Institute, 111 Daedeok-daero 989 beon-gil, Yuseong-gu, Daejeon 34057, Republic of Korea
Abstract
The ternary complexation of calcium uranyl tricarbonate species, CaUO2(CO3)32− and Ca2UO2(CO3)3(aq), which are the predominant U(VI) complexes in groundwater and seawater, was investigated at variable temperatures from 10 to 70 °C. Time-resolved laser fluorescence spectroscopy (TRLFS), calcium ion-selective electrode potentiometry, and ultraviolet/visible (UV/Vis) absorption spectroscopy were complementarily employed to determine the formation constants (log Kx13, x = 1 and 2 for mono- and dicalcium complexes, respectively). 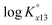 at infinite dilution (zero ionic strength) was determined by correction using specific ion interaction theory (SIT), and an increasing tendency of
at infinite dilution (zero ionic strength) was determined by correction using specific ion interaction theory (SIT), and an increasing tendency of 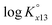 with temperature was observed. In addition, the molar enthalpy of complexation (ΔrHm) was measured by calorimetry at 25 °C. Based on thermodynamic data obtained in this work, the approximation models were examined for the prediction of the temperature effect on the complexation, and the constant enthalpy approximation with the chemical complexation reaction modified to an isoelectric reaction showed a satisfactory prediction of
with temperature was observed. In addition, the molar enthalpy of complexation (ΔrHm) was measured by calorimetry at 25 °C. Based on thermodynamic data obtained in this work, the approximation models were examined for the prediction of the temperature effect on the complexation, and the constant enthalpy approximation with the chemical complexation reaction modified to an isoelectric reaction showed a satisfactory prediction of 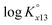 in the temperature range of 10–70 °C. Finally, the results of U(VI) speciation in groundwater indicated that the dominance of calcium uranyl tricarbonate complexes would be weakened at elevated temperatures by the strongly enhanced hydrolysis of U(VI).
in the temperature range of 10–70 °C. Finally, the results of U(VI) speciation in groundwater indicated that the dominance of calcium uranyl tricarbonate complexes would be weakened at elevated temperatures by the strongly enhanced hydrolysis of U(VI).
 You have access to this article
You have access to this article  Please wait while we load your content... About Cited by Related Download options Please wait...
Please wait while we load your content... About Cited by Related Download options Please wait... Supplementary files
- Supplementary information PDF (254K)
Article information
DOI https://doi.org/10.1039/C9DT01174A Article type Paper Submitted 19 Mar 2019 Accepted 12 Apr 2019 First published 13 Apr 2019Download Citation
Dalton Trans., 2019,48, 6942-6950 BibTex EndNote MEDLINE ProCite ReferenceManager RefWorks RISPermissions
Request permissionsFormation of CaUO2(CO3)32− and Ca2UO2(CO3)3(aq) complexes at variable temperatures (10–70 °C)
Y. Jo, A. Kirishima, S. Kimuro, H. Kim and J. Yun, Dalton Trans., 2019, 48, 6942 DOI: 10.1039/C9DT01174A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
Social activity
Search articles by author
Yongheum Jo Akira Kirishima Shingo Kimuro Hee-Kyung Kim Jong-Il Yun  Fetching data from CrossRef. This may take some time to load.
Fetching data from CrossRef. This may take some time to load.
Loading related content 
Spotlight
Advertisements
This website collects cookies to deliver a better user experience. Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa Cookies.Journals
- Current Journals
- Archive Journals
- All Journals
Books
- Browse Books
- Series
- For Authors and Editors
- About
Databases
- Literature Updates
- ChemSpider
- The Merck Index*
- MarinLit
More
- For Members
- For Librarians
- Subscribe
- RSS Feeds
- Blogs
- Chemistry World
- Education in Chemistry
- Open Access
- Historical Collection
Từ khóa » C03 2-
-
Carbonate | CO3-2 - PubChem
-
How To Draw The Lewis Structure Of CO3 2- (Carbonate Ion)
-
Carbonate Ion - How To Draw The Lewis Structure For CO3 2 - YouTube
-
Carbonate - Wikipedia
-
All The C—O Bonds In Carbonate Ion (CO3^2 - ) Are Equal In Length ...
-
716M-LCF12-C03 7-16 DIN Male Connector For 1/2" Coaxial Cable ...
-
[PDF] The Crystal Structure Of BaCa(CO3)2 (barytocalcite)
-
Phase Stability And Dense Polymorph Of The BaCa(CO 3 ) 2 ... - Nature
-
Motor Cửa Cổng Tự động 1 Cánh Tay đòn C03-2 Swing Gate Cao Cấp ...
-
HMC-C032 Datasheet And Product Info - Analog Devices
-
Wrapping CuCo2S4 Arrays On Nickel Foam With Ni2(CO3)(OH)2 ...
-
BaFe[CO3]2, A New Double Carbonate: Synthesis, Structural ...
-
XLC CC-C03 Beluga Bike Rack (for 2 E-Bikes) - Bike-Discount