Calculate The KHC8H4O4 Titer Of 0.125 M KOH. Give The Chemical ...
Có thể bạn quan tâm

Question
Calculate the KHC8H4O4 titer of 0.125 M KOH. Give the chemical reactions involved Calculate the KHC8H4O4 titer of 0.125 M KOH. Give the chemical reactions involvedAdded by Kevin G.
Step 1
10 g/mol H: 1.01 g/mol C: 12.01 g/mol O: 16.00 g/mol Adding these up, we get: 39.10 + 1.01*8 + 12.01 + 16.00*4 = 204.23 g/mol Show more…
Show all steps


Please give Ace some feedback
Your feedback will help us improve your experience
 Calculate the KHC8H4O4 titer of 0.125 M KOH. Give the chemical reactions involved
Calculate the KHC8H4O4 titer of 0.125 M KOH. Give the chemical reactions involved  Powered by NumerAI
Powered by NumerAI 


Muhammad Bin and 77 other Chemistry 101 educators are ready to help you.
Ask a new question
Key Concepts
Recommended Videos
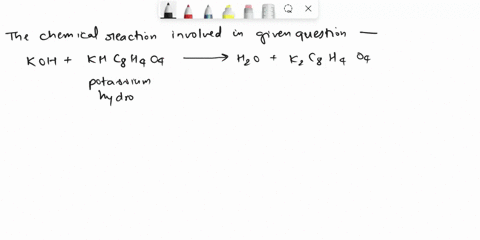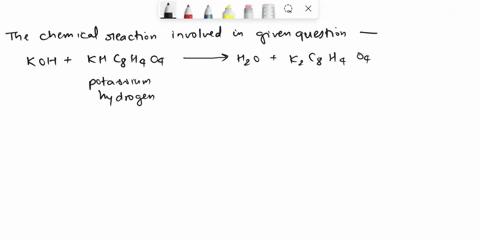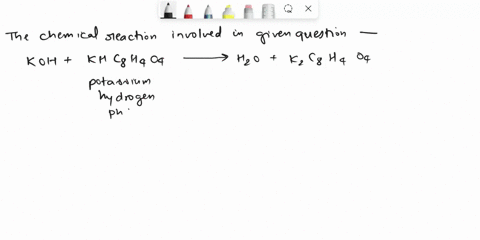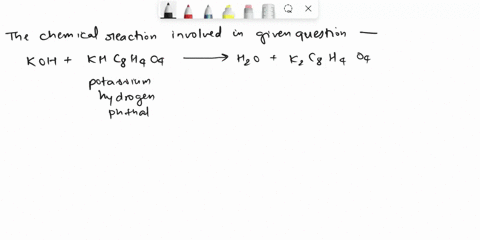
Calculate the Potassium Hydrogen Phthalate (KHC8H4O4) titer of 0.125 M KOH.
Adi S.

The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH solution. What is the concentration of the H3PO4 solution (in M)? Write a balanced equation first. Show each step of this calculation after balancing the equation.
Ronald P.
Determine the percent ionization of a 0.125 $\mathrm{M}$ HCN solution.

Recommended Textbooks

Chemistry: Structure and Properties
Nivaldo Tro 2nd Edition
Chemistry The Central Science
Theodore L. Brown 14th Edition
Chemistry
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste 10th EditionTranscript
What our educators say
 25992 Students Helped in Chemistry 101 “Numerade has a great goal - to increase people's educational levels all around the world. Educators do not complete student's personal homework tasks. We create video tutorials that may be used for many years in the future.”
25992 Students Helped in Chemistry 101 “Numerade has a great goal - to increase people's educational levels all around the world. Educators do not complete student's personal homework tasks. We create video tutorials that may be used for many years in the future.” 

 44601 Students Helped in Chemistry 101 "The format has forced me to think about what knowledge is needed by the student to solve a problem and present it concisely and understandably within the time constraint of the video."
44601 Students Helped in Chemistry 101 "The format has forced me to think about what knowledge is needed by the student to solve a problem and present it concisely and understandably within the time constraint of the video." 

 30417 Students Helped in Chemistry 101 “Explaining topics while I make Numerade videos has helped me deepen my own understanding and come up with new ways to help my students grasp concepts while I'm teaching.”
30417 Students Helped in Chemistry 101 “Explaining topics while I make Numerade videos has helped me deepen my own understanding and come up with new ways to help my students grasp concepts while I'm teaching.” 

 A free answerjust for you
A free answerjust for you Watch the video solution with this free unlock.
View the Answer

Log in to watch this video ...and 100,000,000 more!
PASSWORD
Log in OR Continue with Facebook Continue with Apple Continue with Clever Don't have an account? Sign UpTừ khóa » Khc8h4o4 + Koh
-
KOH + KHC8H4O4 = H2O + K2C8H4O4 - Trình Cân Bằng Phản ứng ...
-
KHC8H4O4 + H2O = KOH + H2C8H4O4 - Trình Cân Bằng Phản ứng ...
-
Solved KHP, Potassium Hydrogen Phthalate (KHC8H4O4), Is - Chegg
-
Balance Chemical Equation - Online Balancer
-
KHP 0.4218 G 중화에 KOH 용액 18.68 ML - 좋은 습관
-
[PDF] . KOH KHC H O K C H O H O + = + - Assignment Expert
-
A Solution Of The Primary Standard Potassium Hydrogen ...
-
Titratable Acidity Of Sebum As Determined By A Micropotentiometric ...
-
A Solution Of The Primary Standard ... - Jiskha Homework Help
-
Tính Nồng độ Mol Của Dd KOH Nếu 0,5480g Chất Góc KHC8H4O4 ...
-
[PDF] CHM 1046 - JOENS
-
Potassium Hydrogen Phthalate - Wikipedia
-
[PDF] Titration Of Aspirin Tablets | Bellevue College