Comparing The E1 And E2 Reactions - Master Organic Chemistry
Có thể bạn quan tâm
E1 versus E2 : Comparing The E1 and E2 Reactions
Now that we’ve gone through the mechanisms of the E1 and E2 reactions, let’s take a moment to look at them side by side and compare them.
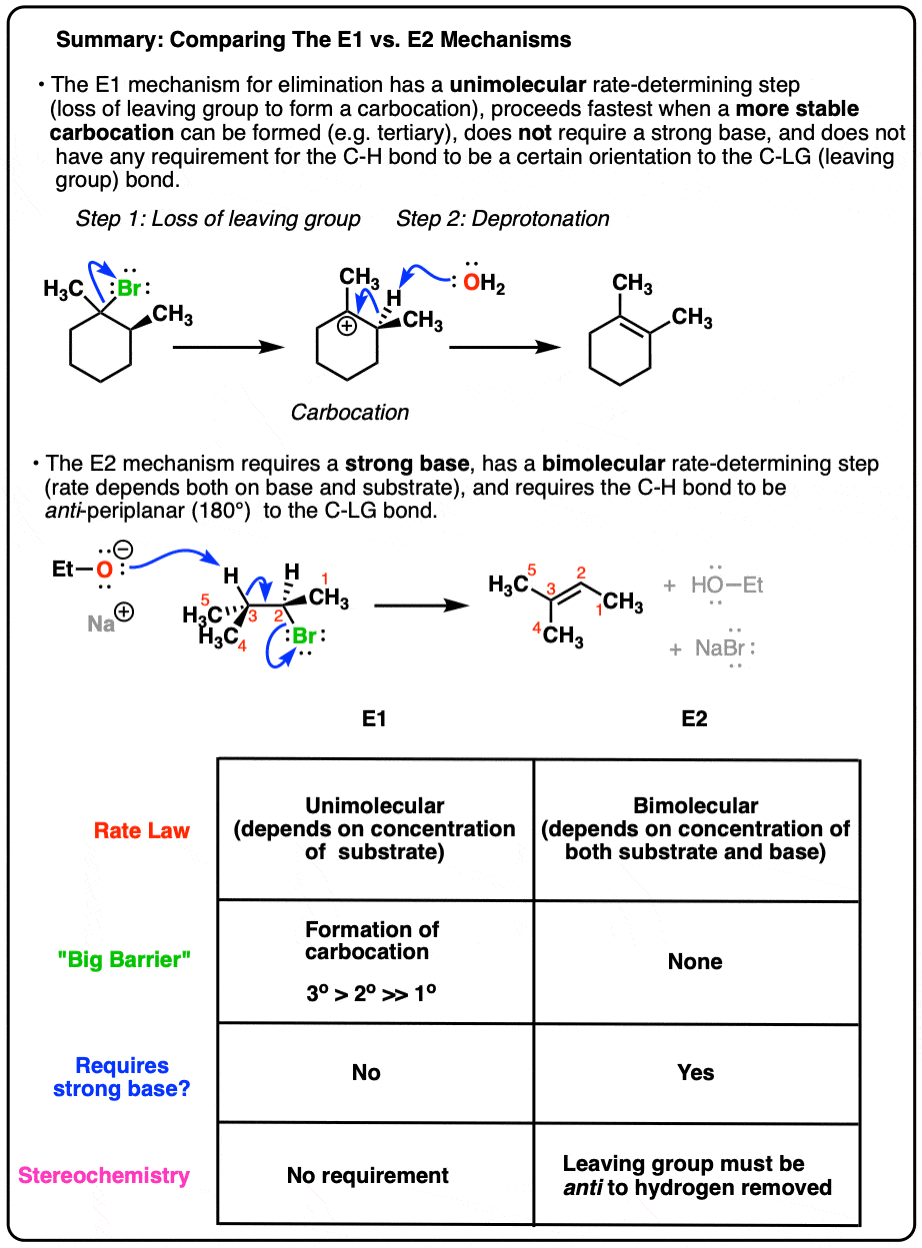
Table of Contents
- Comparing The Mechanism Of The E1 and E2 Reactions
- What Do The E1 and E2 Reactions Have In Common?
- How Are The E1 and E2 Reactions Different?
- E1 vs E2: Why Does One Elimination Give The “Zaitsev” Product, And The Other Elimination Does Not?
- The Key Requirements Of Stereochemistry In The E2 Reaction
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Comparing The Mechanism Of The E1 and E2 Reactions
Here’s how each of them work:
2. What Do The E1 and E2 Reactions Have In Common?
Here’s what each of these two reactions has in common:
- in both cases, we form a new C-C π bond, and break a C-H bond and a C–(leaving group) bond
- in both reactions, a species acts as a base to remove a proton, forming the new π bond
- both reactions follow Zaitsev’s rule (where possible)
- both reactions are favored by heat.
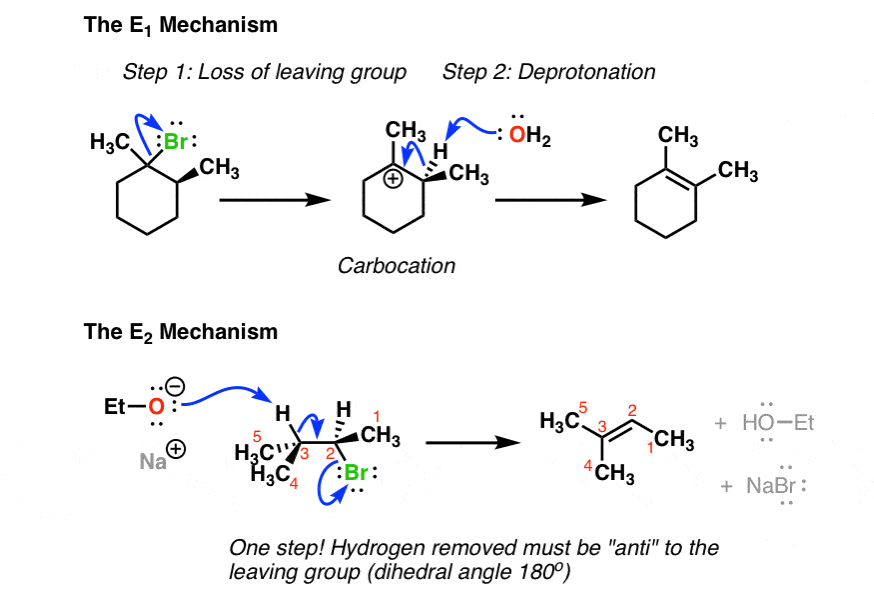
3. How Are The E1 and E2 Reactions Different?
Now, let’s also look at how these two mechanisms are different. Let’s look at this handy dandy chart:
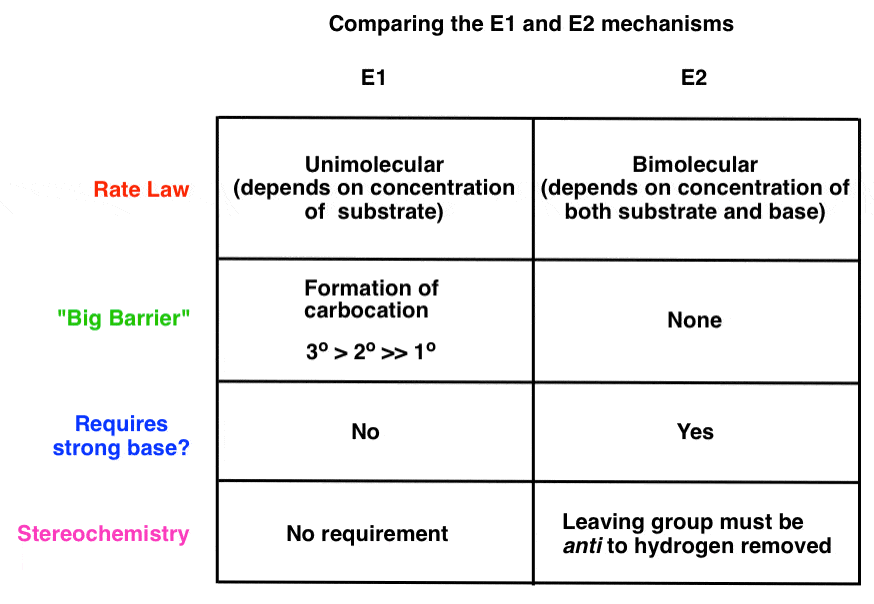
The rate of the E1 reaction depends only on the substrate, since the rate limiting step is the formation of a carbocation. Hence, the more stable that carbocation is, the faster the reaction will be. Forming the carbocation is the “slow step”; a strong base is not required to form the alkene, since there is no leaving group that will need to be displaced (more on that in a second). Finally there is no requirement for the stereochemistry of the starting material; the hydrogen can be at any orientation to the leaving group in the starting material [although we’ll see in a moment that we do require that the C-H bond be able to rotate so that it’s in the same plane as the empty p orbital on the carbocation when the new π bond is formed].
The rate of the E2 reaction depends on both substrate and base, since the rate-determining step is bimolecular (concerted). A strong base is generally required, one that will allow for displacement of a polar leaving group. The stereochemistry of the hydrogen to be removed must be anti to that of the leaving group; the pair of electrons from the breaking C-H bond donate into the antibonding orbital of the C-(leaving group) bond, leading to its loss as a leaving group.
4. E1 vs E2: Why Does One Elimination Give The “Zaitsev” Product, And The Other Elimination Does Not?
Now we’re in a position to answer a puzzle that came up when we first looked at elimination reactions. Remember this reaction – where one elimination gave the “Zaitsev” product, whereas the other one did not. Can you see why now?
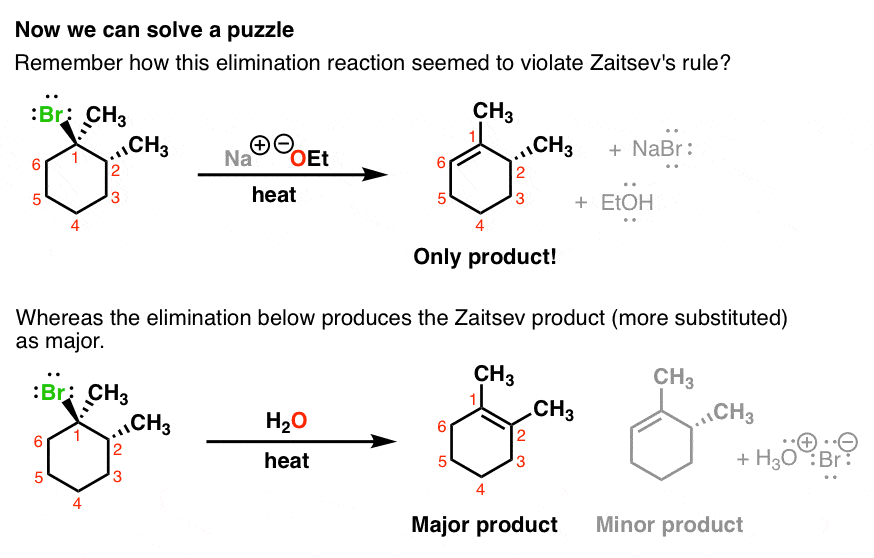
5. The Key Requirements Of Stereochemistry In The E2 Reaction
So what’s going on here?
- The first case is an E2 reaction. The leaving group must be anti to the hydrogen that is removed.
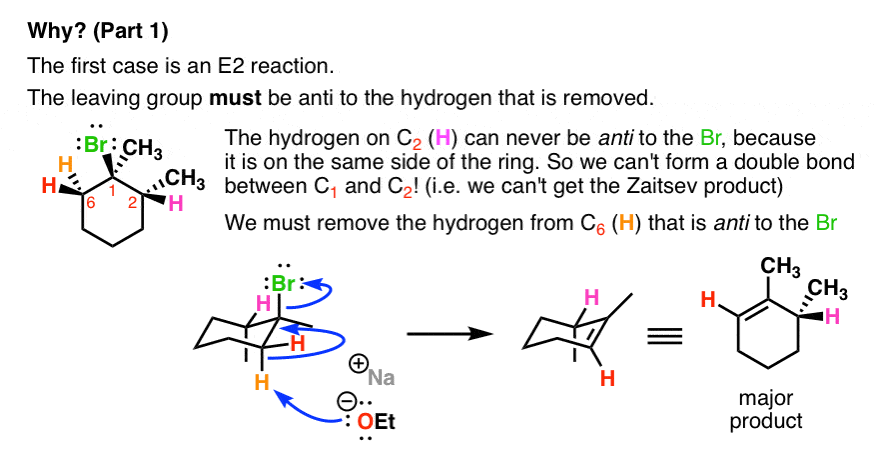
- The second case is an E1 reaction.
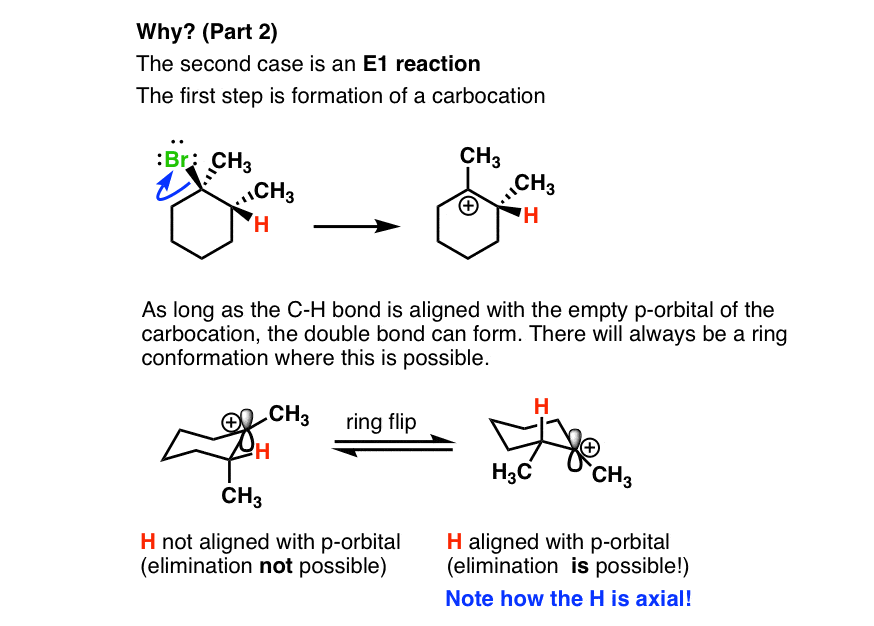
- In our cyclohexane ring here, the hydrogen has to be axial. That’s the only way we can form a π bond between these two carbons; we need the p orbital of the carbocation to line up with the pair of electrons from the C-H bond that we’re breaking in the deprotonation step. We can always do a ring flip to make this H axial, so we can form the Zaitsev product.
- Here’s that deprotonation step:
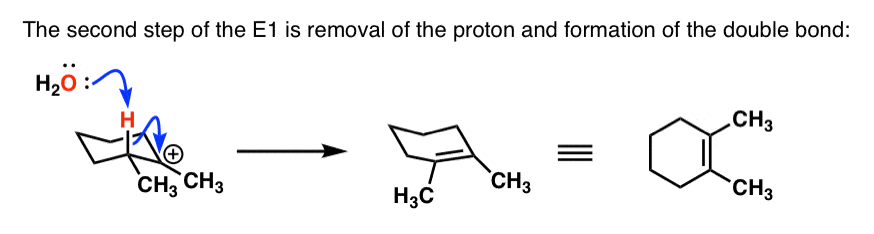
As you can see, cyclohexane rings can cause some interesting complications with elimination reactions! In the next post we’ll take a detour and talk specifically about E2 reactions in cyclohexane rings.
Next Post: Elimination Reactions and Cyclohexane Rings
Notes
Related Articles
- Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings
- E1cB – Elimination (Unimolecular) Conjugate Base
- Elimination Reactions (2): The Zaitsev Rule
- The E2 Mechanism
- The E1 Reaction
- 3 Factors That Stabilize Carbocations
- What makes a good leaving group?
- Elimination Reactions Are Favored By Heat
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
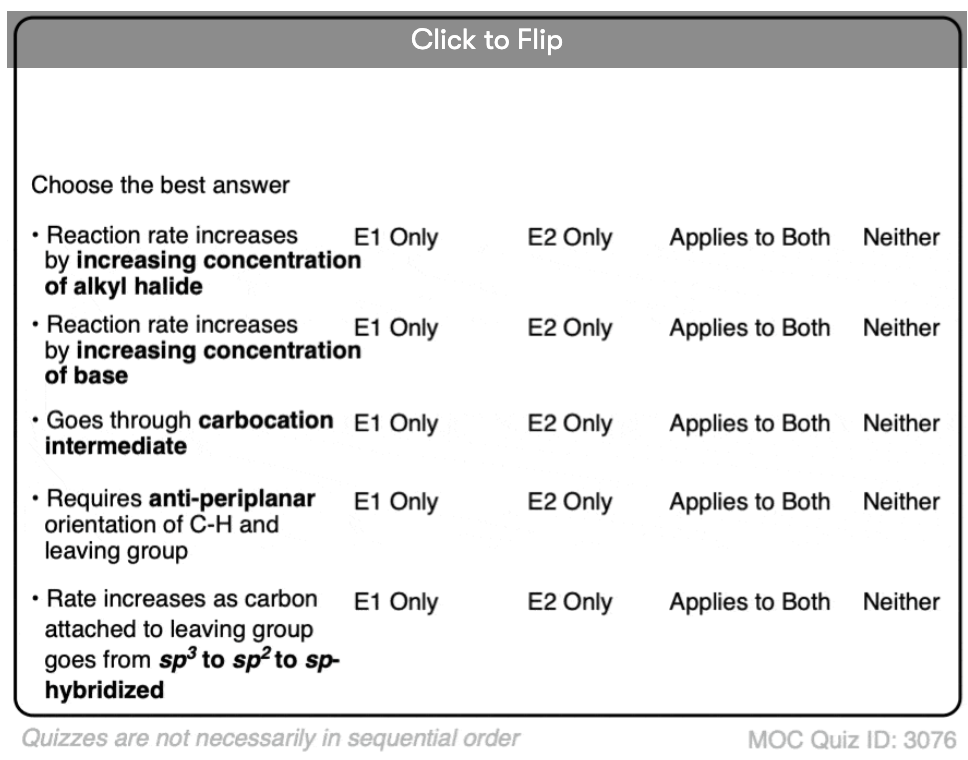
Become a MOC member to see the clickable quiz with answers on the back.
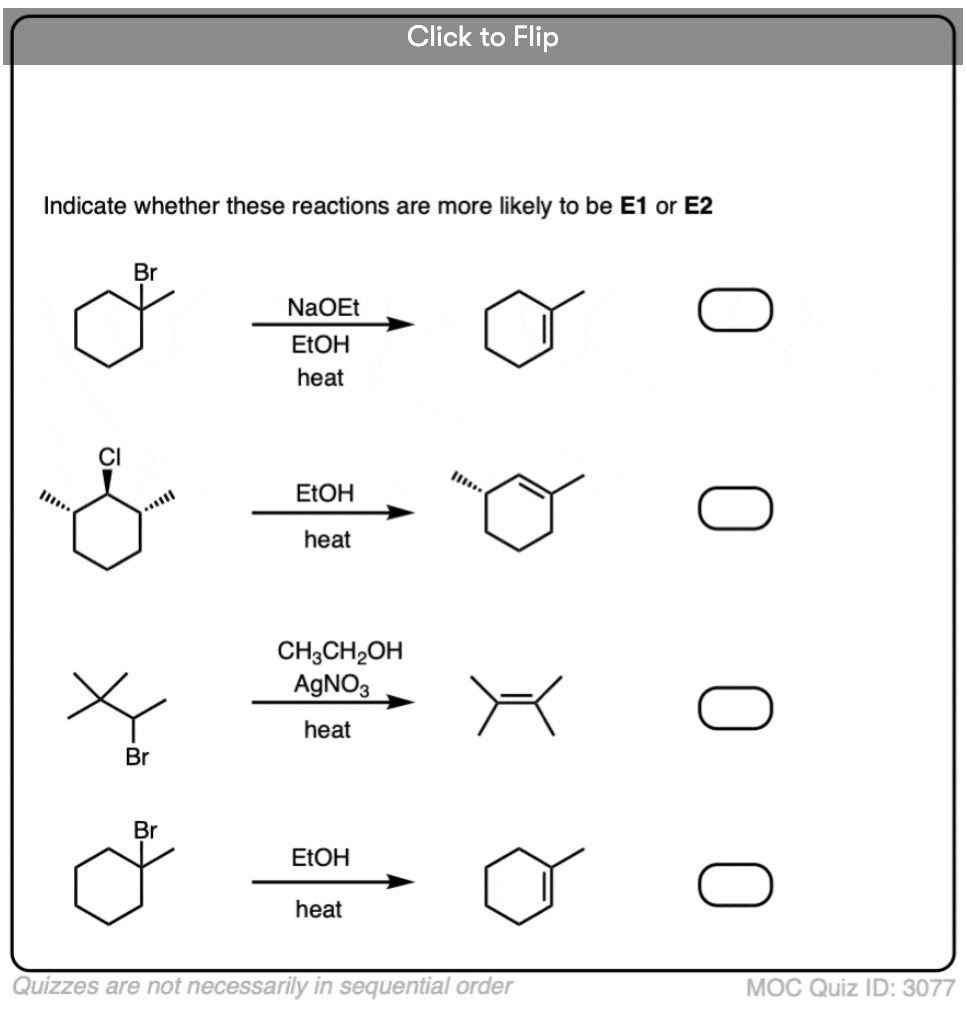
Become a MOC member to see the clickable quiz with answers on the back.
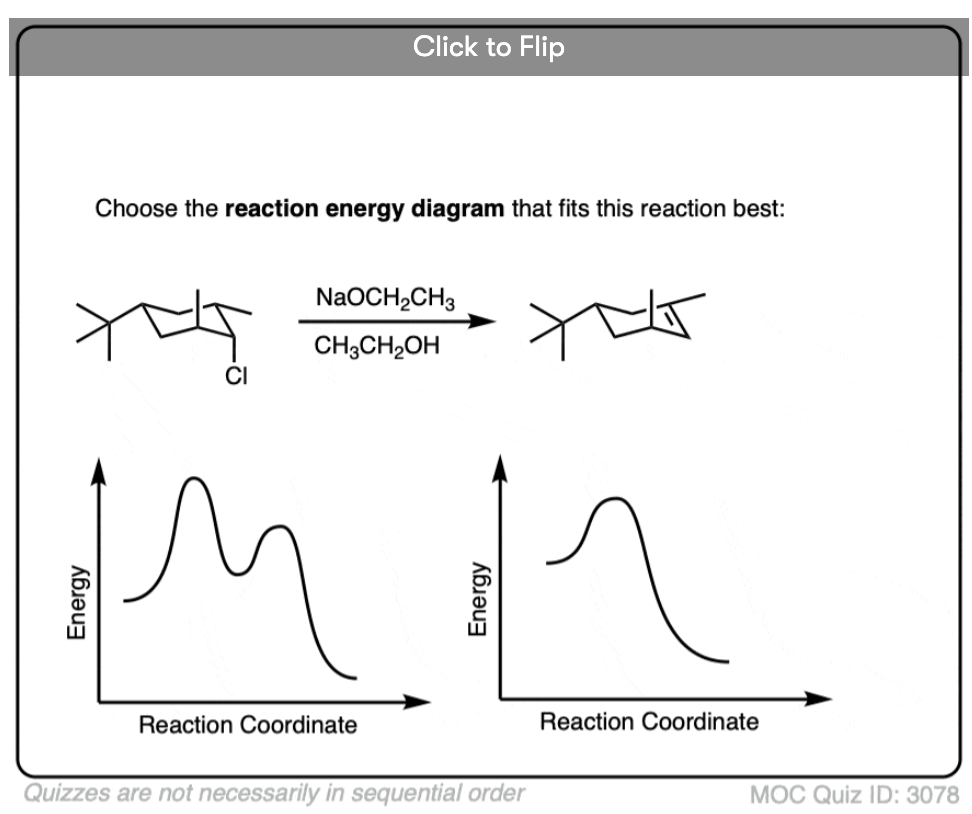
Become a MOC member to see the clickable quiz with answers on the back.
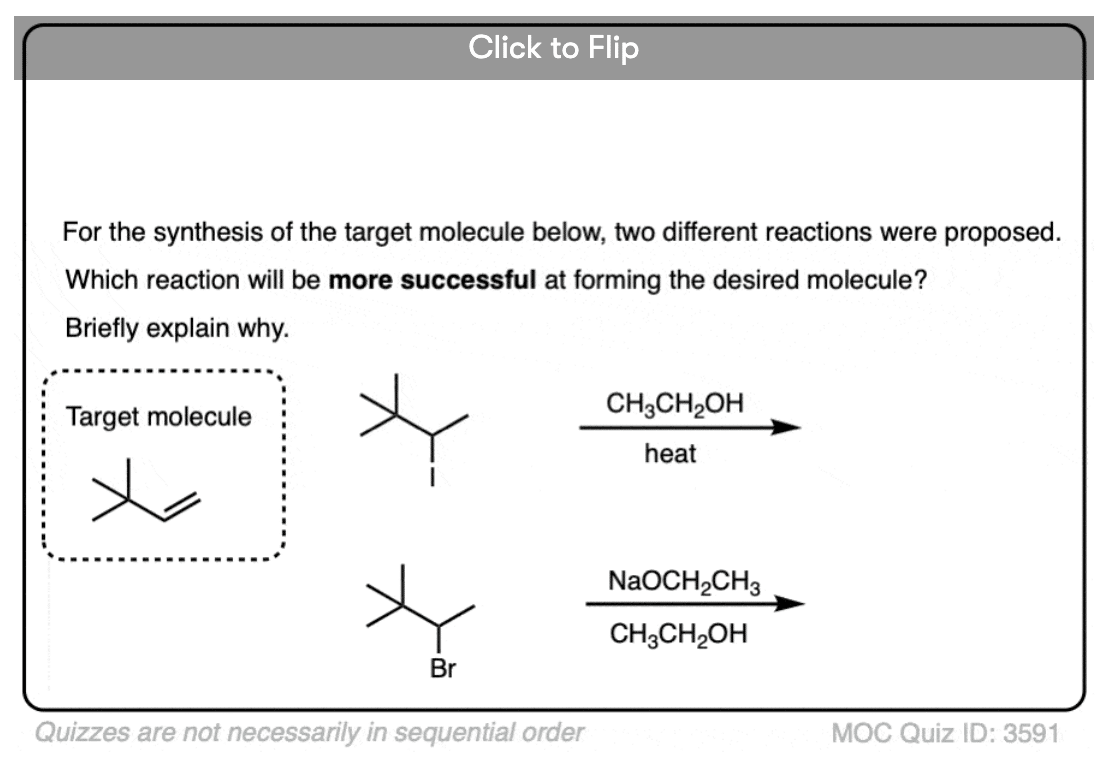
Become a MOC member to see the clickable quiz with answers on the back.
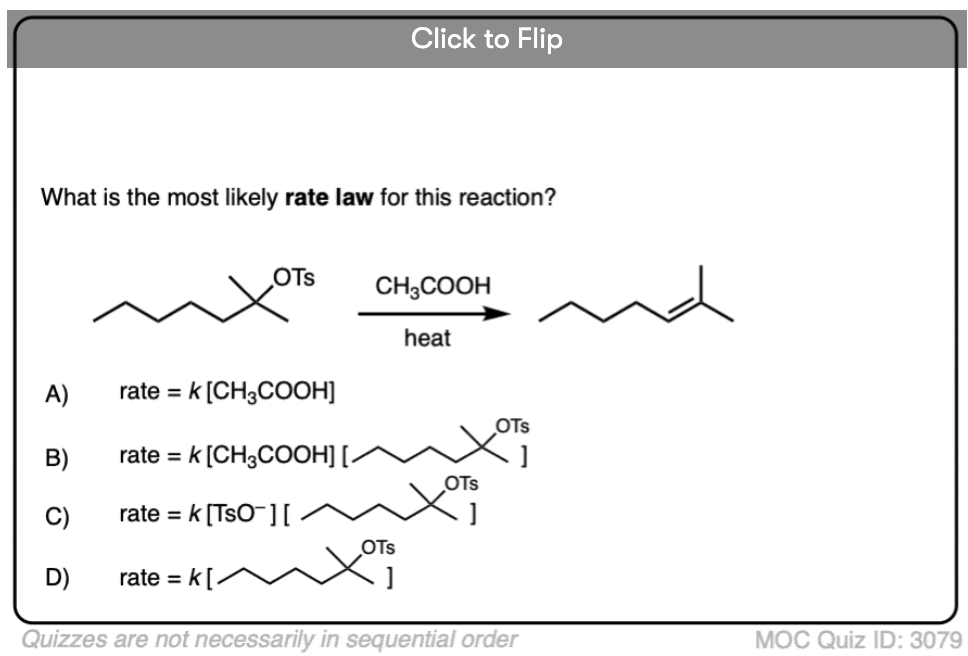
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
- Mechanism of elimination reactions. Part XI. Kinetics of olefin elimination from tert.-butyl and tert.-amyl bromides in acidic and alkaline alcoholic media M. L. Dhar, E. D. Hughes, and C. K. IngoldJ. Chem. Soc. 1948, 2065-2072 DOI: 10.1039/JR9480002065 The E1 reaction is not very useful synthetically for olefin synthesis, because the ratio of elimination to substitution products is substantially lower than in the E2 reaction. For example, solvolysis of t-butyl bromide in dry ethanol only yields 19% isobutylene, whereas 93% yield of the alkene is obtained with 2M ethoxide.
- Mechanisms of elimination reactions. XIII. Effect of base, solvent, and structure on product ratios in elimination reactions of some secondary tosylates Irving N. Feit and William H. SaundersJournal of the American Chemical Society 1970, 92 (6), 1630-1634 DOI: 1021/ja00709a035 Table I in this paper shows that olefin yields under E1 conditions are lower than under E2 conditions. E2 conditions can be promoted by using a strong base, and base strength for the bases employed increases in the order n-butoxide < sec-butoxide < t-butoxide. The effect of reaction conditions on product stereochemistry (trans/cis olefin) is also investigated here.
- Mechanisms of Elimination Reactions. V. Sulfur Isotope Effects in Some Reactions of t-Butyldimethylsulfonium Iodide William H. Saunders and Stuart E. Zimmerman Journal of the American Chemical Society 1964, 86 (18), 3789-3791 DOI: 1021/ja01072a038 This paper features an example of a competition between E2 and E1 that strongly favors E2 when strong base present. In both cases, the reaction rate increases with increasing temperature.
- The mechanism and kinetics of elimination reactions D. Hughes and C. K. Ingold Trans. Faraday Soc. 1941, 37,657-685 DOI: 10.1039/TF9413700657 A review on early investigations of E1 and E2 reactions by Hughes and Ingold, who came up with the terms “E1, E2, SN1, SN2” – these are now called Hughes-Ingold symbols. This review also summarizes the conditions favoring E1/E2 reactions, which are taught to undergraduates the world over every year.
- Eliminations in Cyclic cis‐trans‐Isomers Dr. W. Hückel and Priv.‐Doz. Dr. M. HanackAngew. Chem. Int. Ed. 1967, 6 (6), 534-544 DOI: 10.1002/anie.196705341 Very interesting study comparing the rates of E1 and E2 reactions between cis and trans isomers in a cyclic system. Where E1 and E2 compete, the paper states: “In order that the E2 reaction may be favored as strongly as possible in relation to the El reaction, the alkoxide concentration must be high and the alkyl group of the alkoxide must be as large and as highly branched as possible. Thus for E2 reactions, the order of preference is methanol < ethanol < isoamyl alcohol ≈ isopropyl alcohol < t- butanol < 2-n-butylcyclohexanol.”
Từ khóa » E2 Và E1
-
Sự Khác Biệt Giữa Các Phản ứng E1 Và E2 - Sawakinome
-
Formaldehyde Và Các Cấp độ Phát Thải E0, E1, E2 - Gỗ Minh Long
-
Tiêu Chuẩn Chất Lượng Gỗ Công Nghiệp E2, E1, E0 Là Gì? - FurniBuy
-
Tiêu Chuẩn E0, E1, E2, CARB- P1, CARB- P2, CLASS A, B, C Là Gì?
-
Cơ Chế Phản ứng SN1, SN2, E1 Và E2 | Fantasista's Blog
-
How To Tell If The Mechanism Is E1 Or E2 With Practice Problems
-
Cơ Chế Phản ứng Tách Loại E1 Và E2 - YouTube
-
Tiêu Chuẩn E0, E1, E2, Carb- P1, Carb- P2 Là Gì - OceanB2B
-
Keo E0, E1, E2 Là Gì?
-
Difference Between E1 And E2 In Order Numbers – KBA217986
-
7.18: Comparison Of E1 And E2 Reactions - Chemistry LibreTexts
-
9.9: Comparing The E2 And E1 Reactions Of Alkyl Halides
-
Chỉ Số E Trong Sàn Gỗ Công Nghiệp Và Tác Hại đến Sức Khoẻ Gia đình ...