How Much CO2 Would Be Capture In One Year? - Numerade

Question
1. A fuel has the formula C9H16 and its combustion is complete (i.e. it combines entirely with oxygen to produce CO2 and water). Write down the balanced chemical equation of the combustion. 2. The fuel with formula C9H16 is used in a car that has a fuel efficiency of 20 mpg and is driven for 26,000 miles in one year. If one tree can assimilate 6 kg of CO2 per year, would that tree offset the pollution caused by the car? Show all your calculations. 3. CO2 and H2O combine through the process of photosynthesis to form C6H12O6 and O2. Estimate the consumption of CO2 (in kg per year) by a tree that produces 9 kg of O2 per year. 4. Assuming that the world population is 7 billion and everyone in the planet plants one of the trees that produces 9 kg of O2 per year; how much CO2 would be capture in one year? 1. A fuel has the formula C9H16 and its combustion is complete (i.e. it combines entirely with oxygen to produce CO2 and water). Write down the balanced chemical equation of the combustion. 2. The fuel with formula C9H16 is used in a car that has a fuel efficiency of 20 mpg and is driven for 26,000 miles in one year. If one tree can assimilate 6 kg of CO2 per year, would that tree offset the pollution caused by the car? Show all your calculations. 3. CO2 and H2O combine through the process of photosynthesis to form C6H12O6 and O2. Estimate the consumption of CO2 (in kg per year) by a tree that produces 9 kg of O2 per year. 4. Assuming that the world population is 7 billion and everyone in the planet plants one of the trees that produces 9 kg of O2 per year; how much CO2 would be capture in one year? Show more…Added by Frank H.
Step 1
To balance the combustion reaction of C9H16, we need to find the correct coefficients for O2, CO2, and H2O. The balanced equation is: $$\text{C}_9\text{H}_{16} + \frac{25}{2}\text{O}_2 \rightarrow 9\text{CO}_2 + 8\text{H}_2\text{O}$$ Show more…
Show all steps


Please give Ace some feedback
Your feedback will help us improve your experience
 1. A fuel has the formula C9H16 and its combustion is complete (i.e. it combines entirely with oxygen to produce CO2 and water). Write down the balanced chemical equation of the combustion. 2. The fuel with formula C9H16 is used in a car that has a fuel efficiency of 20 mpg and is driven for 26,000 miles in one year. If one tree can assimilate 6 kg of CO2 per year, would that tree offset the pollution caused by the car? Show all your calculations. 3. CO2 and H2O combine through the process of photosynthesis to form C6H12O6 and O2. Estimate the consumption of CO2 (in kg per year) by a tree that produces 9 kg of O2 per year. 4. Assuming that the world population is 7 billion and everyone in the planet plants one of the trees that produces 9 kg of O2 per year; how much CO2 would be capture in one year?
1. A fuel has the formula C9H16 and its combustion is complete (i.e. it combines entirely with oxygen to produce CO2 and water). Write down the balanced chemical equation of the combustion. 2. The fuel with formula C9H16 is used in a car that has a fuel efficiency of 20 mpg and is driven for 26,000 miles in one year. If one tree can assimilate 6 kg of CO2 per year, would that tree offset the pollution caused by the car? Show all your calculations. 3. CO2 and H2O combine through the process of photosynthesis to form C6H12O6 and O2. Estimate the consumption of CO2 (in kg per year) by a tree that produces 9 kg of O2 per year. 4. Assuming that the world population is 7 billion and everyone in the planet plants one of the trees that produces 9 kg of O2 per year; how much CO2 would be capture in one year?  Powered by NumerAI
Powered by NumerAI 


Amita Prajapat and 73 other Chemistry 101 educators are ready to help you.
Ask a new question
Key Concepts
Recommended Videos
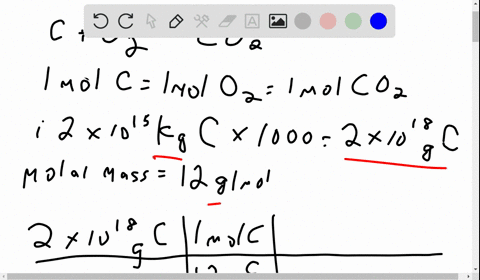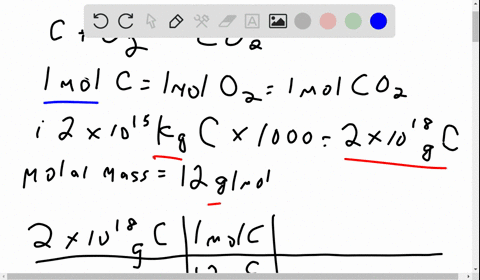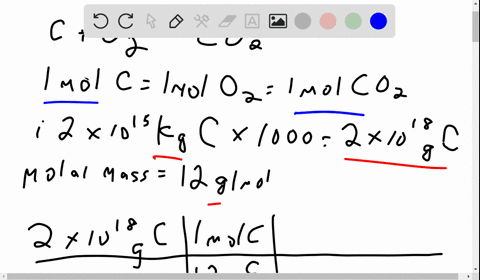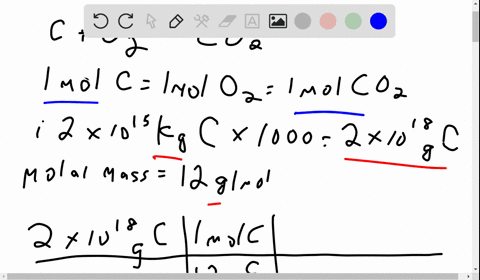
Problem 1: (i) The amount of carbon stored in fossil fuels is 2 x 1015 kg of carbon. If ALL of this carbon were extracted and combusted, how much carbon dioxide would this produce in metric tons of CO2 and in gigatons (Gt) of carbon? Molecular weights: C is 12, molecular oxygen (O2) is 32, and carbon dioxide (CO2) is 44, in equal units. (ii) The mass of molecular oxygen (O2) in the atmosphere is 1.2 x 1018 kg. Would burning ALL fossil fuels in this century consume too much of the atmospheric oxygen? [1 sentence response]
Joseph D.

Global Carbon Dioxide Mass Balance. Recent estimates of carbon dioxide emission and removal rates to and from the atmosphere are summarized in the following table: Carbon Dioxide Sink/Source: Emissions from fossil fuels, Changes in land use, Ocean uptake, Weathering, CO2 fertilization, CO2 release by microorganisms, CO2 removal by photosynthesis, Volcanoes Gigatonnes of Carbon Emitted/Removed (10^9 metric tonnes): 26, 6, -10, -0.7, -10, 440, -440, 0.3 (a) Does your equation describe a steady-state or unsteady-state process? Use this equation to calculate the rate of accumulation of CO2 (not carbon!) in kg/year. (b) If fossil fuel combustion were to increase by +12%, recalculate the rate of accumulation of CO2 in the atmosphere in kg/year. (c) Using the data from the diagram above, calculate the rate-of-change in CO2 concentration in units of ppm per year, and compare this number to the measured rate-of-change of ~2.1 ppm per year. Note: ppm is parts-per-million: i.e. a mole fraction: the moles of CO2 per million moles of atmospheric gases. For this calculation, assume only the first 10 km of atmosphere is well mixed and contains 1.5 x 10^20 moles of gas. (d) Identify at least two natural processes that might release more CO2 as atmospheric CO2 concentration increases.
Adi S.

(a) A methane burner can be represented by the following combustion equation. CH4 + 2O2 . CO2 + 2H2O If one tonne of methane is burnt, how many tonnes of carbon dioxide is produced? (b) If insufficient oxygen is supplied to the methane burner and carbon monoxide is produced instead of carbon dioxide. Derive the combustion equation. (c) The methane burner supplies a total annual household heat requirement of 1800 kWh per year. Calculate the required methane supply in kilograms, assuming that it is operating at 0.21 kg CO2 per kWh. (d) Briefly state five potential benefits of biofuels. (e) A gasifier has been designed to use 20 tonnes an hour of air-dried chopped wood, producing 40 tonnes an hour of fuel gas and the energy content is 5 GJ per tonne. Running the plant requires a constant input of 25 MW supplied by the output gas. If the energy content of air-dried wood is 11 GJ per tonne, what is the overall maximum conversion efficiency of the system? Why is the actual efficiency likely to be lower?
Sri K.
Recommended Textbooks

Chemistry: Structure and Properties
Nivaldo Tro 2nd Edition
Chemistry The Central Science
Theodore L. Brown 14th Edition
Chemistry
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste 10th EditionWhat our educators say
 25992 Students Helped in Chemistry 101 “Numerade has a great goal - to increase people's educational levels all around the world. Educators do not complete student's personal homework tasks. We create video tutorials that may be used for many years in the future.”
25992 Students Helped in Chemistry 101 “Numerade has a great goal - to increase people's educational levels all around the world. Educators do not complete student's personal homework tasks. We create video tutorials that may be used for many years in the future.” 

 44601 Students Helped in Chemistry 101 "The format has forced me to think about what knowledge is needed by the student to solve a problem and present it concisely and understandably within the time constraint of the video."
44601 Students Helped in Chemistry 101 "The format has forced me to think about what knowledge is needed by the student to solve a problem and present it concisely and understandably within the time constraint of the video." 

 30417 Students Helped in Chemistry 101 “Explaining topics while I make Numerade videos has helped me deepen my own understanding and come up with new ways to help my students grasp concepts while I'm teaching.”
30417 Students Helped in Chemistry 101 “Explaining topics while I make Numerade videos has helped me deepen my own understanding and come up with new ways to help my students grasp concepts while I'm teaching.” 

 A free answerjust for you
A free answerjust for you Watch the video solution with this free unlock.
View the Answer

Log in to watch this video ...and 100,000,000 more!
PASSWORD
Log in OR Continue with Facebook Continue with Apple Continue with Clever Don't have an account? Sign UpTừ khóa » C9h16+o2=co2+h2o
-
C9H16 + O2 = CO2 + H2O - Trình Cân Bằng Phản ứng Hoá Học
-
C9H16O2 + O2 = CO2 + H2O - Chemical Equation Balancer
-
Balance Chemical Equation - Online Balancer
-
How To Balance C9H20 + O2 = CO2 + H2O - YouTube
-
Solved 1. A Fuel Has The Formula C9H16 And Its Combustion Is - Chegg
-
Complete Combustion Of A 0.40-mol Sample Of A Hydrocarbon, CxHy ...
-
1. A Fuel Has The Formula C9H16 And Its Combustion Is ... - OneClass
-
[PDF] Combustion Reaction Answers.pdf
-
C9H16O2 B C10H20 C C10H20O D C7H14O3 (C = 12; H = 1; O ...
-
Organic & Biomolecular Chemistry - RSC Publishing