Mole Calculations (n,m,gfm) – National 5 Chemistry Skills & Revision
| Revision Questions | A | B | C | D | E |
| Calculating the mass of one mole (calculating gfm) |  |  |  |  |  |
| Calculating the mass of fractions of a mole. | |||||
| Calculating the number of moles in a given mass. |
| |  |
| In these revision exercises you are asked to use the relationship between number of moles, gram formula mass and the mass of a substance to work out the answer. You should be familiar with the triangle formula and how to manipulate it. All answers have been calculated based on the RAM values detailed in the SQA National 5 databook, published in 2021. |
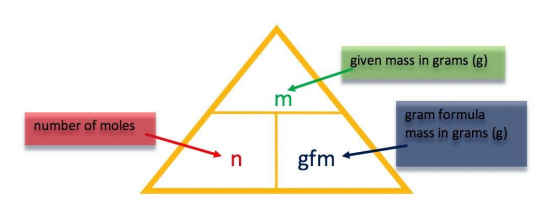 |
Question 1:
Calculate the mass of one mole of the following compounds:
| a) | CaO | b) | KCl | c) | NaBr |
| | | | |||
| d) | LiCl | e) | Fe2O3 | f) | PbBr4 |
| | | | |||
| g) | h) | i) | |||
| | | | |||
| j) | k) | l) | |||
| | | | |||
| m) | n) | o) | |||
| | | |
Question 2
Find the mass of each of the following:
| a) | 0.2 moles of: | b) | 0.3 moles of: | | c) | 0.25 moles of: | ||
| | | | ||||||
| d) | 3 moles of: | e) | 10 moles of: | f) | 0.4 moles of: | |||
| | | | ||||||
| g) | 12 moles of: | h) | 2 moles of: | i) | 0.5 moles of: | |||
| j) | 0.8 moles of: | k) | 1.5 moles of: | l) | 2.4 moles of: |
Question 3
Find the mass of each of the following:
| a) | 2 moles of: | b) | 3 moles of: | c) | 25 moles of: | |||
| | | | ||||||
| d) | 0.3 moles of: | e) | 1.3 moles of: | f) | 2.4 moles of: | |||
| | | | ||||||
| g) | 1.2 moles of: | h) | 0.2 moles of: | i) | 1.5 moles of: | |||
| j) | 4.8 moles of: | k) | 1.5 moles of: | l) | 24 moles of: | |||
| m) | 4.8 moles of: | n) | 1.5 moles of: | o) | 24 moles of: |
Question 4:
Calculate the number of moles in each of the following masses of compounds:
| a) | 2 grams of: | b) | 3 grams of: | c) | 25 grams of: | |||
| d) | 0.3 grams of: | e) | 1.3 grams of: | f) | 2.4 grams of: | |||
| g) | 1.2 grams of: | h) | 0.2 grams of: | i) | 1.5 grams of: | |||
| j) | 4.8 grams of: | k) | 1.5 grams of: | l) | 24 grams of: | |||
| m) | 4.8 grams of: | n) | 1.5 grams of: | o) | 24 grams of: |
Question 5
Calculate the number of moles in the masses of the following compounds:
| a) | 2 grams of: | b) | 3 grams of: | c) | 25 grams of: | |||
| d) | 33 grams of: | e) | 13 grams of: | f) | 24 grams of: | |||
| g) | 12 grams of: | h) | 2 grams of: | i) | 15 grams of: | |||
| j) | 48 grams of: | k) | 1.5 grams of: | l) | 24 grams of: | |||
| m) | 4.8 grams of: | n) | 15 grams of: | o) | 24 grams of: |
Từ khóa » N=m/gfm
-
Convert G F M To N-m | Gram-force Meter To Newton Meters
-
Convert N-m To G F M | Newton Meter To Gram-force Meters
-
BBC Bitesize - The Mole And Concentration Of Solutions
-
PCE TTM 2 Torque Meter (2 Nm / 200 Ncm / 200 Gfm / 20 Lbfin) - EMIN
-
NM Gfm - SoundCloud
-
GFM Là Gì? -định Nghĩa GFM | Viết Tắt Finder
-
NM Gfm Love | Facebook
-
Bơm Tản Nhiệt Nước Custom Bykski Granzon GFM | Shopee Việt Nam
-
National 5: Mole Calculations: Mass = Moles X GFM - YouTube
-
[PDF] Section 1.6 The Mole And Solution Chemistry Summary Notes
-
National 5: Calculating Gram Formula Mass (GFM) - YouTube
-
WR-N/M-GFM
-
[PDF] National 5 CHEMISTRY FORMULAE AND CALCULATIONS