| Pages: 1 2 |
| Author: | Subject: NH3 (aq) or NH4OH |
Mirage Harmless  Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated | ![[*]](images/xpblue/default_icon.gif) posted on 5-2-2012 at 09:22 posted on 5-2-2012 at 09:22 | |
NH3 (aq) or NH4OH So right now, I am trying to figure out weather aqueous ammonia is just an auqeous solution of it, like it should be, or weather it is actually ammonium hydroxide ( which doesn't make much sense). I read in an ancient chem text, that ammonia (household) is really a week solution of NH4OH. I think that that information may be outdated. So aqueous household ammonia is a base, so that means that it must contain an -OH ion. Correct? (I know...Lewis acid blah blah blah...let's keep it basic  ) But isn't gaseous ammonia NH3 a base? Chemically yours Mirage [Edited on 5-2-2012 by Mirage] ) But isn't gaseous ammonia NH3 a base? Chemically yours Mirage [Edited on 5-2-2012 by Mirage] |
| |
Hexavalent International Hazard      Posts: 1564 Registered: 29-12-2011 Location: Wales, UK Member Is Offline Mood: Pericyclic Posts: 1564 Registered: 29-12-2011 Location: Wales, UK Member Is Offline Mood: Pericyclic | ![[*]](images/xpblue/default_icon.gif) posted on 5-2-2012 at 12:43 posted on 5-2-2012 at 12:43 | |
| When ammonia dissolves in water it forms ammonium hydroxide, NH4OH. As a gas, ammonia has the formula NH3. "Success is going from failure to failure without loss of enthusiasm." Winston Churchill |
| |
Pulverulescent National Hazard     Posts: 793 Registered: 31-1-2008 Member Is Offline Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation! Posts: 793 Registered: 31-1-2008 Member Is Offline Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation! | ![[*]](images/xpblue/default_icon.gif) posted on 5-2-2012 at 13:19 posted on 5-2-2012 at 13:19 | |
| Some partial ionisation occurs so it is both solution and base . . . P "I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones" A Einstein |
| |
Mirage Harmless  Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated | ![[*]](images/xpblue/default_icon.gif) posted on 5-2-2012 at 16:09 posted on 5-2-2012 at 16:09 | |
| Ammonia isn't ionic. Correct? The equation would look a bit like an acid dissasociating...correct? What would it be... NH3 + H2O <---> NH4+ + OH- What side would be more favored, I'm expecting the aqueous ammonia to be more favored. Thanks Mirage [Edited on 6-2-2012 by Mirage] |
| |
Engager Hazard to Others    Posts: 295 Registered: 8-1-2006 Location: Moscow, Russia Member Is Offline Mood: Lagrangian Posts: 295 Registered: 8-1-2006 Location: Moscow, Russia Member Is Offline Mood: Lagrangian | ![[*]](images/xpblue/default_icon.gif) posted on 5-2-2012 at 18:26 posted on 5-2-2012 at 18:26 | |
Ammonia in solution is a weak base, and mostly present in undissociated form. Equilibrium constant for reaction NH3 + H2O → NH4(+) + OH(− is 1,8×10−5 at normal conditions, meaning equilibrium is shifted to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase. is 1,8×10−5 at normal conditions, meaning equilibrium is shifted to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase. |
| |
Mirage Harmless  Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated | ![[*]](images/xpblue/default_icon.gif) posted on 5-2-2012 at 20:19 posted on 5-2-2012 at 20:19 | |
I see. well thank you everyone for your help. | Quote: | | Ammonia in solution is a weak base, and mostly present in undissociated form. Equilibrium constant for reaction NH3 + H2O → NH4(+) + OH(− is 1,8×10−5 at normal conditions, meaning equilibrium is shifted to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase. |
|
| |
unionised International Hazard      Posts: 5142 Registered: 1-11-2003 Location: UK Member Is Offline Mood: No Mood Posts: 5142 Registered: 1-11-2003 Location: UK Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 11:19 posted on 6-2-2012 at 11:19 | |
Quote: Originally posted by Hexavalent  | | When ammonia dissolves in water it forms ammonium hydroxide, NH4OH. As a gas, ammonia has the formula NH3. |
Not really, no. Most of the ammonia is just dissolved. Some proton transfer happens so there are a few (about 10 in a million) NH4+ ions, but these are not associated with any particular OH- ions so there's no real NH4OH present. |
| |
Mirage Harmless  Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 12:01 posted on 6-2-2012 at 12:01 | |
Okay, but if then if any NH4+ forms then the corresponding OH- must form. But what your saying is, that it forms in such small amounts that it's like trace compounds. Mirage Quote: Originally posted by unionised  | Quote: Originally posted by Hexavalent  | | When ammonia dissolves in water it forms ammonium hydroxide, NH4OH. As a gas, ammonia has the formula NH3. |
Not really, no. Most of the ammonia is just dissolved. Some proton transfer happens so there are a few (about 10 in a million) NH4+ ions, but these are not associated with any particular OH- ions so there's no real NH4OH present. |
|
| |
Nicodem Super Moderator        Posts: 4230 Registered: 28-12-2004 Member Is Offline Mood: No Mood Posts: 4230 Registered: 28-12-2004 Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 12:20 posted on 6-2-2012 at 12:20 | |
Quote: Originally posted by Mirage  | | Okay, but if then if any NH4+ forms then the corresponding OH- must form. But what your saying is, that it forms in such small amounts that it's like trace compounds. |
What he is saying that there is no such thing as NH4OH. The pKa of the ammonium cation in water is about 8.9, so there is a very, very unfavorable equilibrium for its formation. Yet, that little that forms can only exist as solvated NH<sub>4</sub><sup>+</sup> cations and not as ammonium hydroxide which is an unknown compound anyway. The name "ammonium hydroxide" is an old trivial name that was used in some countries for aqueous solutions of ammonia - it is not a name for a compound corresponding to the actual empirical formula of NH4OH. I hope this is clear enough. …there is a human touch of the cultist “believer” in every theorist that he must struggle against as being unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972) Read the The ScienceMadness Guidelines! |
| |
Mirage Harmless  Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 16:49 posted on 6-2-2012 at 16:49 | |
Crystal clear! Finally! Thanks for everyone's help! Mirage Quote: Originally posted by Nicodem  | Quote: Originally posted by Mirage  | | Okay, but if then if any NH4+ forms then the corresponding OH- must form. But what your saying is, that it forms in such small amounts that it's like trace compounds. |
What he is saying that there is no such thing as NH4OH. The pKa of the ammonium cation in water is about 8.9, so there is a very, very unfavorable equilibrium for its formation. Yet, that little that forms can only exist as solvated NH<sub>4</sub><sup>+</sup> cations and not as ammonium hydroxide which is an unknown compound anyway. The name "ammonium hydroxide" is an old trivial name that was used in some countries for aqueous solutions of ammonia - it is not a name for a compound corresponding to the actual empirical formula of NH4OH. I hope this is clear enough. |
|
| |
GreenD National Hazard     Posts: 623 Registered: 30-3-2011 Member Is Offline Mood: Not really high anymore Posts: 623 Registered: 30-3-2011 Member Is Offline Mood: Not really high anymore | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 17:01 posted on 6-2-2012 at 17:01 | |
| Wow I really thought NH4OH was the main constituent of NH3+H2O... Interesting. |
| |
bahamuth Hazard to Others    Posts: 384 Registered: 3-11-2009 Location: Norway Member Is Offline Mood: Under stimulated Posts: 384 Registered: 3-11-2009 Location: Norway Member Is Offline Mood: Under stimulated | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 17:47 posted on 6-2-2012 at 17:47 | |
| Was told by one of my chemistry teachers that ammonium hydroxide does not exist, and is just a simplification (as mentioned by Nicodem). Ammonium hydroxide does not exist (Read the letter/abstract) Ammonia and "ammonium hydroxide" (I have no acces, this is just the article referred to in the above letter/abstract) Any sufficiently advanced technology is indistinguishable from magic. |
| |
AirCowPeaCock Hazard to Others    Posts: 311 Registered: 9-1-2012 Location: In your nation! Member Is Offline Mood: Hazardous Posts: 311 Registered: 9-1-2012 Location: In your nation! Member Is Offline Mood: Hazardous | ![[*]](images/xpblue/default_icon.gif) posted on 6-2-2012 at 17:49 posted on 6-2-2012 at 17:49 | |
| Why the hell would NH4+ form but not OH-?! Where is it getting its hydrogen? Other ammonia? Like NH4+ NH2-? BOLD |
| |
turd National Hazard     Posts: 800 Registered: 5-3-2006 Member Is Offline Mood: No Mood Posts: 800 Registered: 5-3-2006 Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 01:26 posted on 7-2-2012 at 01:26 | |
| spam reported. |
| |
UKnowNotWatUDo Hazard to Self   Posts: 96 Registered: 30-6-2010 Member Is Offline Mood: No Mood Posts: 96 Registered: 30-6-2010 Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 02:02 posted on 7-2-2012 at 02:02 | |
| Entropy51, so many of your posts lately have been made up of snide, rude, and insulting remarks toward other members. I don't know what your problem is, but a little civility isn't too much to ask for. It's one thing to disagree with someone, but its a whole other thing to simply post for the sake of being a complete and utter asshole. [Edited on 2/7/2012 by UKnowNotWatUDo] |
| |
woelen Super Administrator          Posts: 8146 Registered: 20-8-2005 Location: Netherlands Member Is Offline Mood: interested Posts: 8146 Registered: 20-8-2005 Location: Netherlands Member Is Offline Mood: interested | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 02:57 posted on 7-2-2012 at 02:57 | |
@entropy51:  Stop digging up old and unrelated quotes! GreenD only wrote about the NH3+H2O in this thread. What is written in other threads by him is NOT relevant for this thread. I expected better from you Stop digging up old and unrelated quotes! GreenD only wrote about the NH3+H2O in this thread. What is written in other threads by him is NOT relevant for this thread. I expected better from you  The art of wondering makes life worth living... Want to wonder? Look at https://woelen.homescience.net The art of wondering makes life worth living... Want to wonder? Look at https://woelen.homescience.net |
| |
woelen Super Administrator          Posts: 8146 Registered: 20-8-2005 Location: Netherlands Member Is Offline Mood: interested Posts: 8146 Registered: 20-8-2005 Location: Netherlands Member Is Offline Mood: interested | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 03:04 posted on 7-2-2012 at 03:04 | |
Quote: Originally posted by AirCowPeaCock  | | Why the hell would NH4+ form but not OH-?! Where is it getting its hydrogen? Other ammonia? Like NH4+ NH2-? |
What unionised says is that each NH4(+) ion is not associated to a particular OH(-) ion. You cannot say THIS NH4(+) ion is bound to/associated with THAT OH(-) ion. Of course, for each NH4(+) there also is one OH(-). There will be no NH2(-) in solution. Just look at it as when you dissolve NaCl in water. In the solid, each Na(+) ion is surrounded by a few Cl(-) ions, and these ions are fixed at their position and there is an association between ions. If you could label each ion (e.g. with a name), then you could say that ion with name A is next to ion with name B and this remains true as long as the solid exists. In solution, however, the Na(+)/Cl(-) lattice breaks up and the ions can move through the liquid independent of each other. Ion A can for instance move to one end of the beaker, while ion B moves to the other end of the beaker. You only can say that for each Na(+) ion there is one Cl(-) in solution, but you cannot associate couples of ions to each other. The art of wondering makes life worth living... Want to wonder? Look at https://woelen.homescience.net |
| |
GreenD National Hazard     Posts: 623 Registered: 30-3-2011 Member Is Offline Mood: Not really high anymore Posts: 623 Registered: 30-3-2011 Member Is Offline Mood: Not really high anymore | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 06:55 posted on 7-2-2012 at 06:55 | |
| Thanks for the response on my behalf. The connections he is making in his post are far from reality. It was not my choice to label a NH3 aq solution as sodium hydroxide. So what would the pH of a 30-35% NH3 (aq) solution be? |
| |
ScienceSquirrel International Hazard      Posts: 1863 Registered: 18-6-2008 Location: Brittany Member Is Offline Mood: Dogs are pets but cats are little furry humans with four feet and self determination! Posts: 1863 Registered: 18-6-2008 Location: Brittany Member Is Offline Mood: Dogs are pets but cats are little furry humans with four feet and self determination!  | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 07:12 posted on 7-2-2012 at 07:12 | |
| From this data here, I would guess at just over 12 http://www.airgasspecialtyproducts.com/Libraries/Aqua_Ammoni... |
| |
woelen Super Administrator          Posts: 8146 Registered: 20-8-2005 Location: Netherlands Member Is Offline Mood: interested Posts: 8146 Registered: 20-8-2005 Location: Netherlands Member Is Offline Mood: interested | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 07:32 posted on 7-2-2012 at 07:32 | |
| Yes, pH will be around 12. Normal household ammonia has a pH around 11. This means that the concentration of OH(-) is really low, even in concentrated solutions, in the order of 0.001 M to 0.01 M. The concentration of NH3 is around 3 M for normal household ammonia and the concentrated stuff has around 15 M, or even higher. This nicely shows how weak a base ammonia is. The art of wondering makes life worth living... Want to wonder? Look at https://woelen.homescience.net |
| |
GreenD National Hazard     Posts: 623 Registered: 30-3-2011 Member Is Offline Mood: Not really high anymore Posts: 623 Registered: 30-3-2011 Member Is Offline Mood: Not really high anymore | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 07:33 posted on 7-2-2012 at 07:33 | |
I think I understand. We are not debating that there is a large portion of NH4(+) molecules in solution, right? (.01-.0001M) We are debating that the molecule NH4OH exists. NOW this makes sense. I thought everyone was trying to tell me that NH4+ is not being formed. but it just the complex NH4OH ! Right? This would be the same argument that H4O2 doesn't exist, right - H3O+/OH- molecule? [Edited on 7-2-2012 by GreenD] but it just the complex NH4OH ! Right? This would be the same argument that H4O2 doesn't exist, right - H3O+/OH- molecule? [Edited on 7-2-2012 by GreenD] |
| |
Vikascoder Hazard to Others    Posts: 309 Registered: 28-1-2012 Member Is Offline Mood: No Mood Posts: 309 Registered: 28-1-2012 Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 08:26 posted on 7-2-2012 at 08:26 | |
| Ph refers to power of hydronium ion even the diluted naoh have ph of 14 but its concentration is low so why is the difference of 12 to 11 in ammonia |
| |
ScienceSquirrel International Hazard      Posts: 1863 Registered: 18-6-2008 Location: Brittany Member Is Offline Mood: Dogs are pets but cats are little furry humans with four feet and self determination! Posts: 1863 Registered: 18-6-2008 Location: Brittany Member Is Offline Mood: Dogs are pets but cats are little furry humans with four feet and self determination!  | ![[*]](images/xpblue/default_icon.gif) posted on 7-2-2012 at 09:15 posted on 7-2-2012 at 09:15 | |
Quote: Originally posted by Vikascoder  | | Ph refers to power of hydronium ion even the diluted naoh have ph of 14 but its concentration is low so why is the difference of 12 to 11 in ammonia |
Sodium hydroxide is highly dissociated into solvated sodium and hydroxide ions in water so strong solutions are all around pH14 and even quite dilute solutions are still strong bases, it is only very dilute soltions that are substantially less than that. Ammonia solutions are quite the opposite, dilute solutions are mainly dissolved ammonia with a small proportion of ammonium and hydroxide ions, concentrated ammonia solutions contain much more dissolved ammonia but not much more ammonium and hydroxide ions. I have had a look at this Wikipedia article and I think it is a good explanation; http://en.wikipedia.org/wiki/PH |
| |
arsphenamine Hazard to Others    Posts: 236 Registered: 12-8-2010 Location: I smell horses, Maryland, USA Member Is Offline Mood: No Mood Posts: 236 Registered: 12-8-2010 Location: I smell horses, Maryland, USA Member Is Offline Mood: No Mood | ![[*]](images/xpblue/default_icon.gif) posted on 11-2-2012 at 12:41 posted on 11-2-2012 at 12:41 | |
It looks like the hydrogen bond in un-dissociated NH<sub>4</sub>OH is longer than usual because of the weaker coulombic attraction by the nitrogen. At the MP2/6-311G(3df2p) theory+basis level, the model also doesn't permit a straight line H-bond between the N and O, unlike in water. Here is a model of the HOMO-2 molecular orbitals that show the essential connection and geometry. 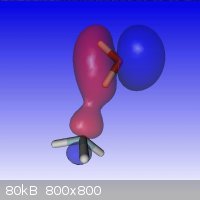 |
| |
Mirage Harmless  Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated | ![[*]](images/xpblue/default_icon.gif) posted on 11-2-2012 at 17:14 posted on 11-2-2012 at 17:14 | |
You lost me at "Columbia attraction"  ... ... Quote: Originally posted by arsphenamine  | | It looks like the hydrogen bond in un-dissociated NH<sub>4</sub>OH is longer than usual because of the weaker coulombic attraction by the nitrogen. At the MP2/6-311G(3df2p) theory+basis level, the model also doesn't permit a straight line H-bond between the N and O, unlike in water. Here is a model of the HOMO-2 molecular orbitals that show the essential connection and geometry. |
|
| |
| Pages: 1 2 |

 Search
Search  FAQ
FAQ  Member List
Member List  Today's Posts
Today's Posts  Stats
Stats 

 Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated
Posts: 49 Registered: 26-1-2012 Location: Canada Member Is Offline Mood: Superheated  ) But isn't gaseous ammonia NH3 a base? Chemically yours Mirage [Edited on 5-2-2012 by Mirage]
) But isn't gaseous ammonia NH3 a base? Chemically yours Mirage [Edited on 5-2-2012 by Mirage] 

 is 1,8×10−5 at normal conditions, meaning equilibrium is shifted to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase.
is 1,8×10−5 at normal conditions, meaning equilibrium is shifted to the left side of eq. above. Aqueous solution of NH3 contains both dissolved NH3 (most part) as well as dissociated species (weak base). Gaseous ammonia isn't the base because terms of acid and base are determined only for solvated environment where ions of H+ or OH- can be formed by iteraction of substance with solvent (solvatation), gas phase at normal conditions can not contain ions so term base can't be used for ammonia in gas phase. 
 Stop digging up old and unrelated quotes! GreenD only wrote about the NH3+H2O in this thread. What is written in other threads by him is NOT relevant for this thread. I expected better from you
Stop digging up old and unrelated quotes! GreenD only wrote about the NH3+H2O in this thread. What is written in other threads by him is NOT relevant for this thread. I expected better from you 

 but it just the complex NH4OH ! Right? This would be the same argument that H4O2 doesn't exist, right - H3O+/OH- molecule? [Edited on 7-2-2012 by GreenD]
but it just the complex NH4OH ! Right? This would be the same argument that H4O2 doesn't exist, right - H3O+/OH- molecule? [Edited on 7-2-2012 by GreenD] 