Nickel Bis(dimethylglyoximate) - Wikipedia
Contents
move to sidebar hide- (Top)
- Article
- Talk
- Read
- Edit
- View history
- Read
- Edit
- View history
- What links here
- Related changes
- Upload file
- Permanent link
- Page information
- Cite this page
- Get shortened URL
- Download QR code
- Download as PDF
- Printable version
- Wikimedia Commons
- Wikidata item
 | |
 | |
| Names | |
|---|---|
| IUPAC name nickel;N-[(Z)-3-nitrosobut-2-en-2-yl]hydroxylamine | |
| Other names Bis(butanedione dioximato)nickel | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider |
|
| EC Number |
|
| PubChem CID |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H14N4NiO4 |
| Molar mass | 288.917 g·mol−1 |
| Appearance | red solid |
| Density | 1.698 g/cm3 |
| Hazards | |
| GHS labelling: | |
| Pictograms |   |
| Signal word | Warning |
| Hazard statements | H315, H317, H319, H335, H351 |
| Precautionary statements | P201, P202, P261, P264, P271, P272, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
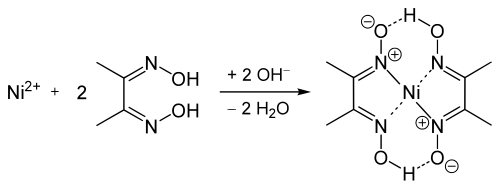
Nickel bis(dimethylglyoximate) is the coordination complex with the formula Ni[ONC(CH3)C(CH3)NOH]2. The compound is a bright red solid. It achieved prominence for its use in the qualitative analysis of nickel.[1]
Structure
[edit]The geometry of the nickel(II) ion is square planar.[2] It is surrounded by two equivalents of the conjugate base (dmgH−) of dimethylglyoxime (dmgH2). The pair of organic ligands are joined through hydrogen bonds to give a macrocyclic ligand. The complex is distinctively colored and insoluble leading to its use as a chelating agent in the gravimetric analysis of nickel.
The use of dimethylglyoxime as a reagent to detect nickel was reported by L. A. Chugaev in 1905.[3]
References
[edit]- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Donald E. Williams; Gabriele Wohlauer; R. E. Rundle (1959). "Crystal Structures of Nickel and Palladium Dimethylglyoximes". J. Am. Chem. Soc. 81 (3): 755–756. doi:10.1021/ja01512a066.
- ^ Tschugaeff, Lev (1905). "Über ein neues, empfindliches Reagens auf Nickel" [About a new, sensitive reagent on nickel]. Berichte der Deutschen Chemischen Gesellschaft (in German). 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
- Nickel complexes
- CS1 German-language sources (de)
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
Từ khóa » Ni(c4h7n2o2)2
-
Ni + C4H8N2O2 = Ni(C4H7N2O2)2 + H - Trình Cân Bằng Phản ứng ...
-
Ni(C4H7N2O2)2 Khối Lượng Mol - ChemicalAid
-
Molar Mass Of Ni(C4H7N2O2)2
-
Thực Tập Hóa Phân Tích - Tài Liệu Text - 123doc
-
Phúc Trình TT Hóa Phân Tích CNHH - Tài Liệu Text - 123doc
-
Thực Tập Hóa Phân Tích - TaiLieu.VN
-
The Formula For A Precipitate Is Ni (C4H7N2O2) 2. What Is ... - Quora
-
PHUC TRINH HOA PHAN TICH 2 - Hóa Học 12 - Nguyễn Thanh Tấn
-
TT. Phân Tích | PDF - Scribd
-
[PDF] BÀI 1: ĐỊNH LƯỢNG NIKEN ( ) ( ) ( )l
-
To Estimate The Amount Of Nickel As Ni Dmg In The Solution Of Nickel …
-
Nickel Dimethylglyoxime | AMERICAN ELEMENTS ®
-
Answered: When The Compound (Ni (C4H7N2O2) 2)… | Bartleby