Safety And Efficacy Of Pomalidomide Plus Low-dose Dexamethasone ...
Abstract
Patients with relapsed and/or refractory multiple myeloma (RRMM) have poor prognosis. The STRATUS study assessed safety and efficacy of pomalidomide plus low-dose dexamethasone in the largest cohort to date of patients with RRMM. Patients who failed treatment with bortezomib and lenalidomide and had adequate prior alkylator therapy were eligible. Pomalidomide 4 mg was given on days 1-21 of 28-day cycles with low-dose dexamethasone 40 mg (20 mg for patients aged >75 years) on days 1, 8, 15, and 22 until progressive disease or unacceptable toxicity. Safety was the primary end point; secondary end points included overall response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Among 682 patients enrolled, median age was 66 years, and median time since diagnosis was 5.3 years. Median number of prior regimens was 5. Most patients were refractory to both lenalidomide and bortezomib (80.2%). Median follow-up was 16.8 months; median duration of treatment was 4.9 months. Most frequent grade 3/4 treatment-emergent adverse events were hematologic (neutropenia [49.7%], anemia [33.0%], and thrombocytopenia [24.1%]). Most common grade 3/4 nonhematologic toxicities were pneumonia (10.9%) and fatigue (5.9%). Grade 3/4 venous thromboembolism and peripheral neuropathy were rare (1.6% each). The ORR was 32.6%, and the median DOR was 7.4 months. Median PFS and OS were 4.6 months and 11.9 months, respectively. We present the largest trial to date evaluating pomalidomide plus low-dose dexamethasone in patients with RRMM, further confirming that this regimen offers clinically meaningful benefit and is generally well tolerated. www.Clinicaltrials.gov identifier NCT01712789.
© 2016 by The American Society of Hematology.
PubMed Disclaimer
Figures
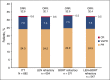
Figure 1
Investigator-assessed response to treatment using…Figure 1
Investigator-assessed response to treatment using IMWG criteria. BORT, bortezomib; CR, complete response; LEN,…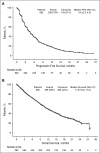
Figure 2
Survival. (A) PFS. (B) OS.Figure 2
Survival. (A) PFS. (B) OS.References
-
- Kumar SK, Lee JH, Lahuerta JJ, et al. International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study [published correction appears in Leukemia. 2012;26(5):1153]. Leukemia. 2012;26(1):149–157. - PMC - PubMed
-
- Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. - PMC - PubMed
-
- Rychak E, Mendy D, Miller K, et al. Overcoming resistance: the use of pomalidomide (Pom) and dexamethasone (Dex) in re-sensitizing lenalidomide (Len)-resistant multiple myeloma (MM) cells [abstract]. Haematologica. 2011;96(s1):s126. Abstract P-328.
-
- Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–2335. - PMC - PubMed
-
- Ocio EM, Fernández-Lázaro D, San-Segundo L, et al. In vivo murine model of acquired resistance in myeloma reveals differential mechanisms for lenalidomide and pomalidomide in combination with dexamethasone. Leukemia. 2015;29(3):705–714. - PubMed
Publication types
- Clinical Trial, Phase III Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Multicenter Study Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Research Support, Non-U.S. Gov't Actions
- Search in PubMed
- Search in MeSH
- Add to Search
MeSH terms
- Adult Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Aged Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Aged, 80 and over Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Antineoplastic Combined Chemotherapy Protocols / administration & dosage* Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Antineoplastic Combined Chemotherapy Protocols / adverse effects Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Dexamethasone / administration & dosage Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Dexamethasone / adverse effects Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Female Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Follow-Up Studies Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Humans Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Male Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Middle Aged Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Multiple Myeloma / drug therapy* Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Thalidomide / administration & dosage Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Thalidomide / adverse effects Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Thalidomide / analogs & derivatives Actions
- Search in PubMed
- Search in MeSH
- Add to Search
Substances
- Thalidomide Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- Dexamethasone Actions
- Search in PubMed
- Search in MeSH
- Add to Search
- pomalidomide Actions
- Search in PubMed
- Search in MeSH
- Add to Search
Associated data
- ClinicalTrials.gov/NCT01712789 Actions
- Search in PubMed
- Search in ClinicalTrials.gov
LinkOut - more resources
Full Text Sources
- Elsevier Science
- Europe PubMed Central
- PubMed Central
- Silverchair Information Systems
Other Literature Sources
- scite Smart Citations
Medical
- ClinicalTrials.gov
- MedlinePlus Health Information
Từ khóa » Cc-4047-mm-010
-
Evaluation Of Safety Of Pomalidomide In Combination With ...
-
MTD, Safety, And Efficacy Of Pomalidomide (CC-4047) Alone Or With ...
-
ICTRP Search Portal
-
No - EU Clinical Trials Register
-
[PDF] 204026Orig1s000 - Accessdata.
-
Pomalidomide Plus Low-dose Dexamethasone Versus High ... - PubMed
-
Genetic And Transcript Changesin Cereblon In IMiD-Treated ...
-
Large-Scale Analysis Of Relapsed/Refractory ... - ASH Publications
-
Large-Scale Analysis Of Relapsed/Refractory Multiple Myeloma ...
-
[PDF] (S)AE Instructie CC-4047-MM-010, STRATUS
-
Large-Scale Analysis Of Relapsed/Refractory Multiple Myeloma ...
-
CC-4047-MM-015- Pomalidomide For Relapsed And Refractory MM ...
-
Multiple Myeloma Patient Tumors With High Levels Of Cereblon ...