EduRev Less EduRev Explore Exams

Explore Courses UPSC Exam UPSC Test Series Free Notes EduRev Infinity Get the App Login Join for FreeSign in Open App
NEET Exam > NEET Questions > The maximam volume at sTP is occupied by 1 12... Start Learning for Free The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?
![]() Clear all your NEET doubts with EduRev Clear all your NEET doubts with EduRev | |
Most Upvoted AnswerThe maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 m...Maximum Volume at STPAt STP (Standard Temperature and Pressure), the temperature is 273 K and the pressure is 1 atmosphere (atm). At this condition, one mole of any ideal gas occupies 22.4 L of volume. Therefore, the maximum volume at STP can be calculated for different substances.1. 12.8g of SO2To calculate the maximum volume occupied by 12.8g of SO2, we need to convert the mass to moles using the molar mass of SO2, which is 64 g/mol. moles of SO2 = 12.8 g / 64 g/mol = 0.2 molNow, using the ideal gas law, we can calculate the volume occupied by 0.2 mol of SO2 at STP.V = nRT/P = (0.2 mol)(0.0821 L atm/mol K)(273 K) / 1 atm = 4.4 L2. 6.02x10^23 molecules of CH4To calculate the maximum volume occupied by 6.02x10^23 molecules of CH4, we need to convert the number of molecules to moles using Avogadro's number.moles of CH4 = 6.02x10^23 / 6.022x10^23 mol^-1 = 1 molTherefore, the maximum volume occupied by 1 mol of CH4 at STP is 22.4 L.3. 0.5 mol of NO2To calculate the maximum volume occupied by 0.5 mol of NO2, we can use the ideal gas law.V = nRT/P = (0.5 mol)(0.0821 L atm/mol K)(273 K) / 1 atm = 11.2 L4. 1 gram molecule of CO2A gram molecule of CO2 is the amount of CO2 that has a mass of one mole. The molar mass of CO2 is 44 g/mol. Therefore, the mass of one gram molecule of CO2 is 44 g. To calculate the maximum volume occupied by one gram molecule of CO2 at STP, we can use the ideal gas law.moles of CO2 = 1 g / 44 g/mol = 0.023 molV = nRT/P = (0.023 mol)(0.0821 L atm/mol K)(273 K) / 1 atm = 0.5 LConclusionIn conclusion, the maximum volume occupied at STP depends on the amount and type of gas. The maximum volume is calculated using the ideal gas law, which relates the volume, pressure, temperature, and amount of gas. Therefore, the maximum volume occupied at STP by the substances given are:1. 12.8g of SO2 = 4.4 L2. 6.02x10^23 molecules of CH4 = 22.4 L3. 0.5 mol of NO2 = 11.2 L4. 1 gram molecule of CO2 = 0.5 LAnswered by Dr. Ritesh Malhotra ·
View profileCommunity AnswerThe maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 m...
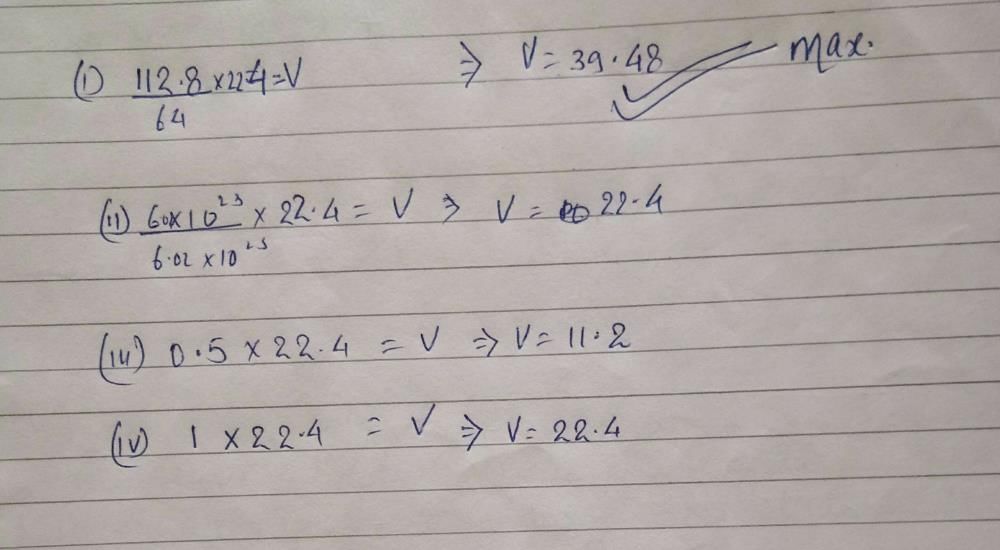
Share your Answer
![]() | Explore Courses for NEET exam | ![]() |
Top Courses for NEETView all
![]()
Daily Test for NEET Preparation
![]()
4 Months Preparation for NEET
![]()
NEET Mock Test Series - Updated 2026 Pattern
![]()
Biology Class 11
![]()
Topic-wise MCQ Tests for NEET
Top Courses for NEET
View all
![]() Question Description
Question Description The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? for NEET 2026 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? covers all topics & solutions for NEET 2026 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?.Solutions for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? in English & in Hindi are available as part of our courses for NEET. Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.Here you can find the meaning of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? defined & explained in the simplest way possible. Besides giving the explanation of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?, a detailed solution for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? has been provided alongside types of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? theory, EduRev gives you an ample number of questions to practice The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? tests, examples and also practice NEET tests.
![]() | Explore Courses for NEET exam | ![]() |
Top Courses for NEET
Explore Courses
 Join the Discussion on the App for FREE
Join the Discussion on the App for FREERelated Content
Signup for Free!Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.Start learning for Free10M+ students study on EduRev×
Share your doubts
Submitx![]() For Your Perfect Score in NEETThe Best you need at One PlaceStart Your Infinity Experience
For Your Perfect Score in NEETThe Best you need at One PlaceStart Your Infinity Experience

View answers on AppView all answers and join this discussion on the EduRev AppView in AppNot Now × Signup to see your scores go up within 7 days!Access 1000+ FREE Docs, Videos and TestsContinue with GoogleTakes less than 10 seconds to signup
 Less EduRev Explore Exams
Less EduRev Explore Exams  Explore Courses UPSC Exam UPSC Test Series Free Notes EduRev Infinity Get the App Login Join for FreeSign in Open App NEET Exam > NEET Questions > The maximam volume at sTP is occupied by 1 12... Start Learning for Free The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?
Explore Courses UPSC Exam UPSC Test Series Free Notes EduRev Infinity Get the App Login Join for FreeSign in Open App NEET Exam > NEET Questions > The maximam volume at sTP is occupied by 1 12... Start Learning for Free The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?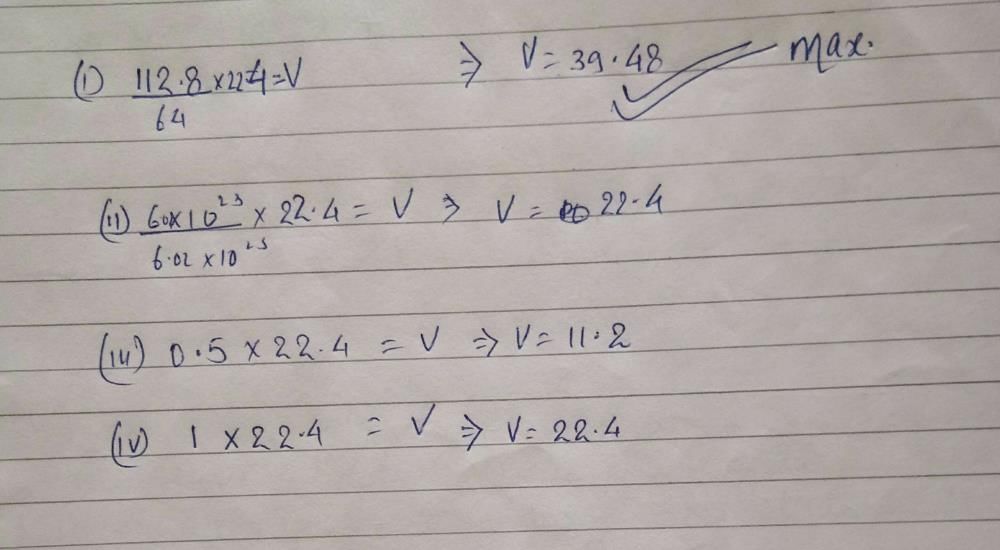 Share your Answer
Share your Answer![]()
![]()
![]()
![]()
![]()
![]() Question Description The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? for NEET 2026 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? covers all topics & solutions for NEET 2026 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?.Solutions for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? in English & in Hindi are available as part of our courses for NEET. Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.Here you can find the meaning of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? defined & explained in the simplest way possible. Besides giving the explanation of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?, a detailed solution for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? has been provided alongside types of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? theory, EduRev gives you an ample number of questions to practice The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? tests, examples and also practice NEET tests.
Question Description The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? for NEET 2026 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? covers all topics & solutions for NEET 2026 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?.Solutions for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? in English & in Hindi are available as part of our courses for NEET. Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.Here you can find the meaning of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? defined & explained in the simplest way possible. Besides giving the explanation of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?, a detailed solution for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? has been provided alongside types of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? theory, EduRev gives you an ample number of questions to practice The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? tests, examples and also practice NEET tests. Join the Discussion on the App for FREE
Join the Discussion on the App for FREE View answers on AppView all answers and join this discussion on the EduRev AppView in AppNot Now × Signup to see your scores go up within 7 days!Access 1000+ FREE Docs, Videos and TestsContinue with GoogleTakes less than 10 seconds to signup
View answers on AppView all answers and join this discussion on the EduRev AppView in AppNot Now × Signup to see your scores go up within 7 days!Access 1000+ FREE Docs, Videos and TestsContinue with GoogleTakes less than 10 seconds to signup  Explore Courses UPSC Exam UPSC Test Series Free Notes EduRev Infinity Get the App Login Join for FreeSign in Open App NEET Exam > NEET Questions > The maximam volume at sTP is occupied by 1 12... Start Learning for Free The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?
Explore Courses UPSC Exam UPSC Test Series Free Notes EduRev Infinity Get the App Login Join for FreeSign in Open App NEET Exam > NEET Questions > The maximam volume at sTP is occupied by 1 12... Start Learning for Free The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? Clear all your NEET doubts with EduRev
Clear all your NEET doubts with EduRev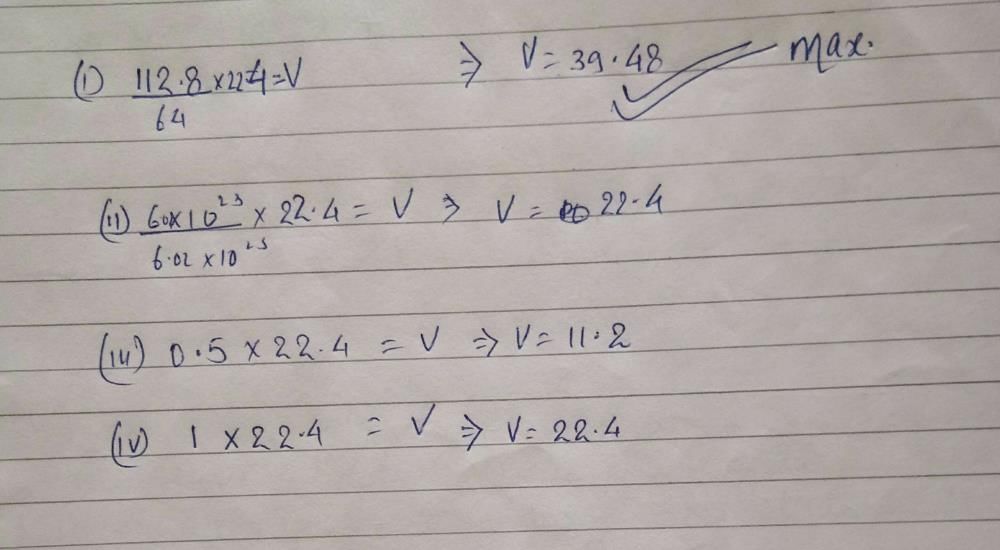 Share your Answer
Share your Answer






 Question Description The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? for NEET 2026 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? covers all topics & solutions for NEET 2026 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?.Solutions for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? in English & in Hindi are available as part of our courses for NEET. Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.Here you can find the meaning of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? defined & explained in the simplest way possible. Besides giving the explanation of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?, a detailed solution for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? has been provided alongside types of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? theory, EduRev gives you an ample number of questions to practice The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? tests, examples and also practice NEET tests.
Question Description The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? for NEET 2026 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? covers all topics & solutions for NEET 2026 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?.Solutions for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? in English & in Hindi are available as part of our courses for NEET. Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.Here you can find the meaning of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? defined & explained in the simplest way possible. Besides giving the explanation of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ?, a detailed solution for The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? has been provided alongside types of The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? theory, EduRev gives you an ample number of questions to practice The maximam volume at sTP is occupied by 1 12.8g of so2 2 6.02x10'23 molecules of ch4 3 0.5 mol of No2 4 1grm molecule of co2 ? tests, examples and also practice NEET tests.

 Join the Discussion on the App for FREE
Join the Discussion on the App for FREE![]() For Your Perfect Score in NEETThe Best you need at One PlaceStart Your Infinity Experience
For Your Perfect Score in NEETThe Best you need at One PlaceStart Your Infinity Experience